Document Type : Original Research Paper
Authors
- Lydia Rohmawati 1
- Lytha Rizqika Lailia 2
- Nugrahani Primary Putri 2
- Munasir Nasir 2
- Darminto Darminto 3
1 Department of physics, Faculty of Mathematics and Sciences, Universitas Negeri Surabaya, Surabaya, Indonesia
2 Department of Physics, Faculty of Mathematics and Sciences, Universitas Negeri Surabaya, Surabaya, Indonesia
3 Department of Physics, Faculty of Science and Data Analytics, Teknologi Sepuluh Nopember, Surabaya, Indonesia
Abstract
ZnO nanoparticles can be used as a photocatalyst in waste treatment because they have good photodegradation. Synthesis of ZnO nanoparticles using green synthesis method from pineapple peel extract, whose results were characterized by XRD (Xray Diffraction), FTIR (Fourier Transform Infra-red), FESEM (Field Emission-Scanning Electron Microscopy), TEM (Transmission Electron Microscopy), Raman spectroscopy, photoluminescence, and a photocatalytic activity assay. This research showed that ZnO nanoparticles had wurtzite phase, alcohol functional groups, and phenol O-H, C=C alkenes, C-O, C-N, and Zn-O. ZnO nanoparticles had a particle size of 20.04 nm, a spherical shape, and a band gap energy of 3.28 eV. The Raman active mode E2(High) at 439.05 cm-1 confirmed the formation of pure phase wurtzite. Photoluminescence results indicated that two emission peaks at 392.07 nm and 595.07 nm were associated with defects such as oxygen and zinc vacancies. The results of the photocatalytic effectiveness test showed the highest percent degradation value of 99.86% at 180 minutes using UV light.
Graphical Abstract
Highlights
Keywords
INTRODUCTION
Over the last two years, industrial developments have created chemical wastes polluting water and the surrounding environment. Pollution is known to cause high toxicity [1]. Chemical waste contains toxic dyes and is very dangerous, made from azo compounds and their derivatives, as well as the benzene groups, such as methylene blue [2]. Methylene blue is carcinogenic and can be a source of disease if it is in the environment for a long time [3]. Organic dyes are synthetic materials that the environment cannot degrade so these compounds can accumulate in nature [4]. With the existence of chemical waste, which is an environmental issue, a method is needed to develop water purification technology that is implemented in different ways, for example, purifier, distillation, adsorption, flocculation, and photocatalyst [5]. Photocatalyst is a possible waste treatment method because the process is simple and effective in cleaning biological and chemical contaminants [6,7].
Moreover, organic photoactive compounds in this photocatalytic process can degrade organic pollutants where the dissolved organic material can function self-cleaning on contaminated surfaces through photodegradation of species that are active when exposed to sunlight [8]. Photocatalytic degradation, which belongs to the advanced oxidation process, is an energy-saving process for degradation processes, where nanostructured photocatalysts play an essential role in promoting accelerated decomposition by generating reactive species such as hydroxyl radical (•OH) and superoxide (•O2-) using suitable light and energy [9]. Besides that, the photodegradation of organic dyes can increase due to the presence of hole scavengers [10].
Photocatalytic materials with good photodegradation potential are semiconductors. One of the catalysts used is the semiconductor ZnO (zinc oxide), which has an energy gap ranging from 3.32 to 3.37 eV with a nanostructure so that it can be applied mainly for wastewater treatment. The material has a high redox capability, resulting in considerable electron mobility and better catalytic performance [11]. This semiconductor can produce active substances for photocatalytic applications after irradiating at a specific wavelength [12,13]. ZnO photocatalyst has versatile properties, easy synthesis, and a relatively low cost [14]. In addition, ZnO in powder form also has unique properties, namely being non-toxic, biocompatible, and having high catalytic activity in degrading toxic pollutants [15]. Parvizi et al. (2019) reported that photocatalytic activity is closely related to optical absorption [16]. Among different semiconductors, ZnO nanoparticles have shown higher efficiency in the photocatalytic degradation of some organic dyes than TiO2 [17]. That is because the number of photogenerated electron-hole recombinations decreases [18]. The catalytic activity of ZnO can be maintained, is stable, and does not experience a significant decrease when reused [19].
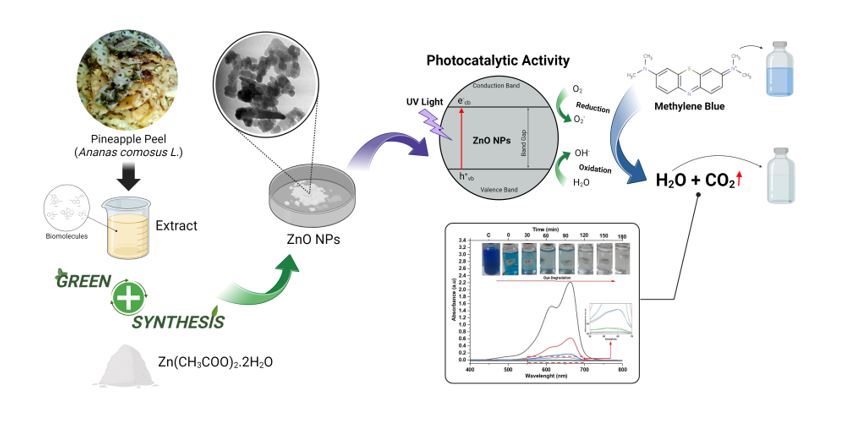
ZnO nanoparticles can be extracted from several plants, including pineapple peel extract. Pineapple peel contains bioactive compounds such as saponins, flavonoids, tannins, anthocyanins, vitamin C, carotenoids, and the enzyme bromelain [20]. Mirgane et al. (2020) reported that ZnO nanoparticles from pineapple peel could be used for wastewater treatment [21]. The study showed that 99.60% of the dyestuffs degraded from textile industry wastewater within 900 minutes. In this study, the characteristics of optical properties such as photoluminescence were not reported, even though this photoluminescence technique can be used to determine the characteristics of photogenerated recombination of electron and hole pairs. Dewi and Rohmawati (2022) reported that ZnO nanoparticles with the wurtzite phase were successfully extracted from pineapple skin, where the manufacturing method used green synthesis [22]. Biosynthesis of ZnO nanoparticles is more provident and environmentally affable than chemical or physical synthesis methods [23].
Based on the information from the research result above, the application of ZnO from pineapple peel extract has never been reported for the degradation of methylene blue so this research will discuss in more detail the characteristics of ZnO nanoparticles from pineapple peel extract as photocatalytics. Apart from that, this research also discusses catalyst stability and the reuse of ZnO nanoparticle catalysts in the degradation of methylene blue dye. The results of this study will provide solutions to the textile industrial waste problem by using natural materials for effective photocatalytic degradation, especially for organic dye waste.
MATERIAL AND METHODS
Materials
The tools and materials used were pineapple skin (Ananas comosus L.), distilled water, zinc acetate dihydrate (Merck), NaOH 1M (Merck), methylene blue (Merck), mortal pestle, spatula, digital balance, oven, beaker, centrifuge, stirrer, sonicator, measuring cup, petri dish, funnel, pH paper, filter paper Whatman, and aluminum foil.
Green synthesis of ZnO nanoparticles
The synthesis of ZnO nanoparticles was done by peeling the pineapple fruit, taking the inner skin pericarp, and then rinsing it with distilled water. As much as 10 grams of pineapple peel were mashed using a mortal pestle, then the results were put into a 250 mL beaker, and 100 mL of distilled water was added. The solution was stirred at 300 rpm at 75°C for 1 hour. After that, the solution was filtered, and a yellow filtrate was obtained. The result is added 10 mL of distilled water and 4 grams of zinc acetate dihydrate, then sonicated at 40°C for 1 hour. Furthermore, 100 mL of 1 M NaOH was added to mold a milky white solution, then centrifuged at 3000 rpm, and the results were parched at 120°C for 12 hours. Here is a chemical reaction during green synthesis using NaOH solution.
Characterization of ZnO nanoparticles
XRD (X-ray Diffraction) characterization was used to determine the formation of the primary phase in the ZnO sample. The type of XRD tool used was Smartlab Rigaku with a Cu anode radiation source, 40 kV, 30 mA, 0.01º/minute with a CuKα wavelength of 1.54056 Å and an angle (2θ) of 5º-90º. The phases contained in the sample could be identified through qualitative analysis using the software Match!. FTIR characterization (Fourier Transform Infrared Spectroscopy) type Thermoscientific Nicolet Is-10 was used to identify groups of chemical bonds owned by the test sample with absorption peaks at wave numbers 4000-500 cm-1. The morphology of the sample in the form of the synthesized grain shape could be identified using the FE-SEM tool (Field Emission-Scanning Electron Microscopy) at 20000 times magnification with the type Jeol JSM-IT200 equipped with EDX (Energy Dispersive X-Ray). The synthesized nanoparticles’ shape, structure, and distribution could be known from the TEM characterization results (Transmission Electron Microscopy) of the Hitachi H7700, where the analytical method used ImageJ software. The typical vibration mode of the material could be identified using the Raman spectroscopy characterization tool iHR320 HORIBA and a laser source with a wavelength of 532 nm. The interaction between the monochromatic light radiation source and the sample caused scattering due to the vibration of the identified compounds. The results of the Raman Spectroscopy test were in the form of a relationship graph between intensity and wavenumber. The sample vibration peaks were identified at wave numbers 2000-265 cm-1. With a 325 nm laser beam and a 1200 g/mm grating, a Horiba Scientific PL (Photoluminescence Microspectrometer) was used to identify structural defects in materials such as oxygen vacancies. The data from the test was in the form of a relationship graph intensity, and the wavelength (nm) ranges from 300-600 nm, which was then plotted using the graph software Origin 8.0; then, the results were matched with data from the journal references. The UV-Vis characterization of the Hitachi UH-5300 spectrophotometer was used to measure the absorbance of a sample with a wavelength of 200-800 nm. Based on the data from this characterization, the band gap analysis could be done using the Tauc plot analysis.
Photocatalytic Activity
Before adding the ZnO nanoparticle powder catalyst to the Methylene Blue (MB) solution, it is necessary to make a 30 ppm MB solution first, namely, 30 ml of MB solution (100 ppm) mixed with 100 ml of distilled water, then stir until evenly mixed. After that, 10 mg of ZnO nanoparticle powder was added to the solution and stirred using a magnetic stirrer for 30 minutes at 300 rpm. After that, it was irradiated using a UV Sterilizer lamp (10 W with 30 mW/cm-2) with time intervals of 30, 60, 90, 120, 150, and 180 minutes. The solution resulting from the irradiation was centrifuged for 15 minutes at a speed of 3000 rpm so that it was homogeneous and no lumps of catalyst were found in the solution. After that, the solution was put into a glass vial (10 ml) for UV-vis characterization.
The solution MB absorbance value, degraded with a catalyst, was measured using a UV-Vis spectrophotometer. The test results were plotted using the graphing software Origin 8.0 to obtain the curve for the linear regression equation.
% Degradation is the percentage of degradation, C0 is the initial concentration of methylene blue without irradiation, and Ct is the final concentration of methylene blue at time t after the photocatalytic process [24].
RESULT AND DISCUSSION
XRD Characterization Results
The diffraction pattern of the synthesized sample can be seen in Fig. 3, which is then analyzed using the software Match!. The synthesized sample has a phase wurtzite formed at an angle of 2θ of 36.253º. At this angle, it has the highest diffraction intensity with crystal orientation (101), following JCPDS card number 01-079-0206, and following the research results of Ahmad et al. (2019) and Sari et al. (2021), namely at an angle of 36.245º [25] and 36.28º [26]. Other diffraction peaks indicate phase wurtzite at angles of 31.768º, 34.422º, 36.253º, 47.539º, 56.594º, 62.858º, 66.374º, 67.947º, 69.085º, 72.568º, 76.959º, 81.387º and 89.613º with miller indices (100), (002), (101), (102), ( 110), (103), (200), (112), (201), (004), (202), (104), and (203). The diffraction pattern of Fig. 3 is also not found in impurity, so it can be said that all of the synthesized samples are the wurtzite phase of ZnO.
FTIR Characterization Results
The functional groups of the synthesized ZnO nanoparticles were identified in the FTIR spectra with wave numbers of 4000-500 cm-1 (Fig. 4). The absorption peak at wave number 3350 cm-1 showed the presence of alcohol and phenol O-H bonds, and this was following the results of Nagajyoti et al. (2015) which were at wave number 3380 cm-1 [27]. The alkene chemical bond C=C was detected at a wave number of 1653 cm-1. Bond vibrations of C=O with carboxylic acid groups were indicated at wave number 1549 cm-1.
In addition, at 1438 cm-1 denotes the C-O functional group, and at 1082 cm-1, a C-N vibration occurs. At the absorption peak of 866 cm-1, the conformation of Zn tetrahedral coordination occurs, following the results by Jurablu et al. (2015), which were at wave number 875 cm-1 [28]. Functional groups of Zn-O are found at wave numbers 782, 688, and 548 cm-1. Basri et al.(2020) reported in their research that the functional groups of ZnO nanoparticles were at wave numbers of 800-400 cm-1 [29]. Detailed information about the absorption peaks in the samples can be seen in Table 1.
FE-SEM Characterization Results
The morphology of the synthesized ZnO nanoparticles was determined by FE-SEM analysis at 20,000x magnification, as shown in Fig. 5. FE-SEM shows that the synthesized samples have a polyhedral shape with a uniform size distribution, although the sample dimensions cause some agglomeration. The content and percentage by weight of elements can be known from the results of the EDX spectrum, where peaks appear corresponding to the ZnO content, namely the presence of O and Zn elements with percentages of 19.7% and 80.3%, respectively. On the contrary, this EDX analysis demonstrated the pureness of the synthesized ZnO nanoparticles, which comprise a high composition of Zn and O elements [33].
TEM Characterization Results
Particle size distribution can be known using ImageJ, namely by considering 100 items per sample. Fig. 6 shows that the synthesized samples have a uniform polyhedral shape, low agglomeration, and an average particle size of 20.04 nm. Based on the particle size, the synthesized ZnO sample can be said to be a nanoparticle because the grain size is less than 100 nm. A regular and uniform polyhedral surface can be associated with the increased photocatalytic ability of ZnO nanoparticles [34].
Raman Spectroscopy Characterization Results
Data from spectroscopic Raman characterization results are shown in Fig. 7. Raman from ZnO nanoparticles with structure wurtzite pertains to the space group C46v (P63 mc), generating a vibrational pattern in the Brillouin zone [35].
A1 + 2B1 + E1 +2E2
Known 2B1 is a stationary phonon, while the other mode is a Raman active phonon [35]. Mode A1 and E1 are polar modes divided into optical transverse (TO) and optical longitudinal (LO). Mode E2 is a non-polar mode consisting of E2(low) and E2(high) phonons. Mode E2(low) is a vibrational mode of the zinc lattice, and mode E2(high) is an arrangement of wurtzite, a ZnO nanoparticle lattice that includes shifts of oxygen atoms [36]. Mode E2(Low) at 100.26 cm-1 and mode E2(High) at 439.05 cm-1 are characteristic phonons of wurtzite-phase ZnO nanoparticles that have good crystallinity in the ZnO structure and are related to the vibration of zinc oxygen in the crystal lattice [37]. Mode LO is at 232.85 cm-1 A1 and 618.997 cm-1 E1. Mode A1 (LO) and E1 (LO) are associated with interstitial oxygen or zinc vacancies. At 203.536 cm-1, 2E2(Low) is an overtone of E2(Low), and another at 335.04 cm-1 E2(Low) - E2(High), a combination of E2(Low) and E2(High) [38].
The Raman spectrum also shows broad peaks ranging from 653.82, 1346.16, and 1418.34 cm-1, which belong to the multiphonon process due to the combination of optics. A mode vibrational activity A1 (LO) + E2(High) at 928.09 cm-1 is a multiphoton scattering mode. The vibration mode is detected at 582.65 cm-1 of which E1 (LO) is activated; this mode indicates the process of multiphonon resonance in the ZnO nanoparticle structure. Optical phonon activation is associated with interstitial oxygen defect formation in Zn [39]. For a more detailed explanation, it can be seen in Table 2 regarding the summit of the Raman spectrum on the synthesized samples.
PL Characterization Results
Photoluminescence (PL) of ZnO nanoparticles was performed to come across structural imperfection primarily owing to Zn and oxygen vacancies. Fig. 8 shows that synthesized samples’ PL exhibits two emission peaks, such as emission peak at 392.07 nm is related to Near Band Emission (NBE) [42], and the emission peak is located at 595.07 nm and is related to Deep Level Emission (DLE) namely oxygen and Zn vacancies. The UV emission band, known as the NBE band gap of ZnO nanoparticles, is caused by the excitonic recombination of holes in the valence band and electrons in the conduction band through the exciton collision process.
Meanwhile, visible light emission, known as DLE, is caused by crystal defects [43]. DLE can be attributed to the recombination of electrons and holes at the deep band gap level caused by the presence of various crystal defects in the lattice of ZnO nanoparticles such as zinc interstitial (Zni), zinc vacancies (VZn), oxygen vacancies (VO), interstitial oxygen (Oi), and oxygen antisite (OZn). The vacancies of Zn, oxygen, and interstitial atoms in the synthesized samples can be observed through PL measurements and Raman spectroscopy. Various types of defects, specifically VO (oxygen vacancies), are known to exist in three configurations such as neutral oxygen vacancies (VO), single ionized oxygen vacancy (VO+), and double ionized oxygen vacancy (VO2+). Based on the emission spectrum in Fig. 8, it is appreciated that the presence of oxygen vacancies can increase photocatalytic activity. It is related to the oxygen vacancy in the sample, which will cause the positive free charge (hole) to attract oxygen more quickly and form H2O2, producing hydroxyl radicals.
Photocatalytic ZnO Nanoparticles
The photocatalytic performance of ZnO nanoparticles is highly dependent on their electronic band structure and bandgap energy. The results of the UV-vis characterization using the Tauc plot analysis are in Fig. 9. It is known that the synthesized ZnO nanoparticles have an energy gap of around 3.28 eV; this is smaller than 3.37 eV, which is the standard ZnO energy gap. This means that the photocatalyst activity of the ZnO nanoparticles is efficient because the generated electrons are more easily excited and produce more hydroxyl radicals. This hydroxyl radical is used as an oxidizer for methylene blue (MB) compounds to become harmless substances. The absorption peak of ZnO nanoparticles was found at a wavelength of 380 nm in the UV region; this result follows the study by Alharshan et al. [44].
The UV-vis absorption spectrum of ZnO nanoparticles towards MB dye with varying times during the photodegradation process up to 180 minutes is shown in Fig. 10. There was a difference in the uptake of methylene blue dye (control) in the presence of ZnO nanoparticle catalyst without UV light irradiation, where the uptake of methylene blue dye decreased from 2.217 to 0.625 a.u. The absorption intensity of MB dye gradually decreases with the length of time of UV irradiation, especially at a wavelength of 664 nm, which is indicated by the fading of the dye MB until it becomes clear, which indicates degradation of the MB dye molecules. That is because the longer the radiation exposure time, the color of the solution will fade, making it easier to reach the photocatalyst. It is known that the degradation of MB solution dye without UV irradiation is 71.81%. When exposed to UV irradiation for 30, 60, 90, 120, 150, and 180 minutes, the MB dye was degraded with percentages of 92.47, 97.20, 97.25, 99.32, 99.68 and 99.86%, respectively (Fig. 11).
The duration of irradiation can affect the degradation percentage of methylene blue by a photocatalyst (Fig. 11). This is due to the interaction between the dye molecules and the photocatalyst surface. The longer the irradiation time, the more dyes can be degraded until they reach their optimum time because of the large number of •OH radicals formed. The duration of irradiation indicates the interaction between the photocatalyst and UV light to produce •OH radicals and the interaction between •OH radicals and methylene blue dyes. The OH radical plays a role in the decomposition of methylene blue to be a safe compound, so the longer the irradiation time, the more •OH radicals are formed and the greater the percent degradation value produced. With an increase in the photon energy produced, more •OH is produced and formed. It is a potent oxidizing agent that can be used to degrade MB dyes [45].
In this research, ZnO nanoparticles synthesized using the green synthesis method showed good photocatalytic performance, especially in degrading MB dye under UV light irradiation, which is known to have the highest degradation efficiency of 99.86% in a short time, 180 minutes. Several studies on the photodegradation activity of MB with ZnO nanoparticles using several methods are shown in Table 3. Based on the results of several researchers, all previously reported that ZnO nanoparticle photocatalysts required a longer reaction time to degrade the dye, and the degradation rate was relatively low. Thus, the ZnO nanoparticles synthesized in this study showed excellent photocatalytic activity with increasing irradiation time. The ZnO nanoparticle catalyst degraded the MB dye, and the MB dye solution was almost completely degraded.
Catalyst stability and catalyst reuse were carried out using five experimental cycles, namely using UV irradiation for 180 minutes, the results of which are shown in Fig. 12. ZnO nanoparticles as a catalyst remained active against UV light, and the absorption of MB dye remained stable with a degradation value of 99.86%. It confirms that the synthesized ZnO nanoparticles can be applied effectively for environmental applications, namely as a photocatalyst in textile industry waste processing.
CONCLUSION
ZnO nanoparticles were successfully synthesized using the method of green synthesis and have a phase wurtzite, polyhedral shape with an average particle size of 20.04 nm, have alcohol and phenol O-H functional groups, C=C alkene, C-O, C-N, and Zn-O. In addition, ZnO nanoparticles have a Raman active optical phonon mode E2(High) at 439.05 cm-1, which confirms the formation of the phase wurtzite. The photoluminescence characterization showed two emission peaks at 392.07 nm and 595.07 nm. These two peaks are associated with structural defects such as oxygen vacancies and Zn. ZnO nanoparticles have an energy gap of 3.28 nm with an absorption peak at a wavelength of 380 nm, which is in the UV region. The results of the photocatalytic effectiveness test showed that the percent degradation value of ZnO nanoparticles from pineapple peel extract with a catalyst mass of 10 mg and 30 ppm methylene blue is capable of degrading dyes by 99.86% within 180 minutes. Thus, the longer the irradiation time, the higher the percent degradation value of methylene blue, such as organic dyes. ZnO nanoparticles as a catalyst have stability in absorbing methylene blue dye and remain active under UV light even though the ZnO nanoparticle catalyst has been reused after five cycles. Thus, ZnO nanoparticles from pineapple peel are effective as photocatalytics and can be applied in processing organic dye waste.
ACKNOWLEDGEMENT
The author would like to thank the Physics Laboratory and Integrated Laboratory of Universitas Negeri Surabaya, Central Laboratory Physics Research of the National Research and Innovation Agency (BRIN), University of Indonesia Chromatography Laboratory (UI), and Gajah Mada University Inorganic Chemistry Laboratory (UGM) for the support of laboratory.
CONFLICT OF INTEREST
The authors declare no conflict of interest.