Document Type : Original Research Paper
Authors
- Shobha Musmade 1
- Dinesh Punja Hase 2
- Amit S Waghmare 3
- Kailas R Kadam 1
- Jayshree Khedkar 4
- Anil G Gadhave 1
- Kanhaiyalal S Bhavsar 5
- Vaishali Dattatray Murade 1
1 Padmashri Vikhe Patil’s Arts, Science and Commerce College, Pravaranagar, Loni KD., Ahmednagar, MS, India
2 Amrutvahini College of Pharmacy, Sangamner Ahmednagar, MS, India
3 ASC College, Satral, Ahmednagar, MS, India
4 Shri Anand College, Pathardi, Ahmednagar, MS, India
5 MGSM’s Arts, Science and Commerce College, Chopda, Jalgaon, India
Abstract
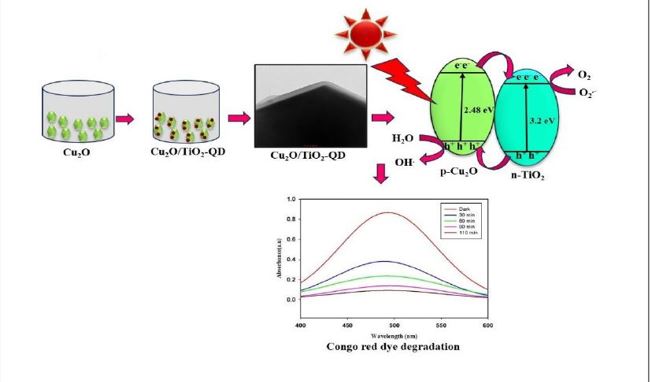
In the present work, truncated octahedron and octahedron shaped Cu2O and Cu2O/TiO2-QD composite have been synthesized using precipitation method and used as a photocatalyst. Synthesized material was characterized by using various analytical techniques- XRD, SEM, TEM and UV-Visible DRS. TEM images clearly shows that the TiO2 was highly dispersed and firmly anchored on single crystals of copper oxide in Cu2O/TiO2-QD composite and also confirmed the truncated octahedron and octahedron shapes of copper oxide. The excellent performance of synthesized Cu2O/TiO2-QD photocatalyst has been proved the maximum degradation (89.00%) of Congo red dye at pH=6. The effect of various parameters in the dye degradation such as influence of pH, amount of photocatalyst, concentration of dye and reusability of the photocatalyst has been studied. The highest degradation rate was found with concentration of Congo red dye 9 mg/L, 150mg/L of Cu2O/TiO2-QD, and 110 min. Thus, the photocatalytic performance of Cu2O/TiO2-QD composite revealed the excellent and effective degradation of Congo-red dye.
Graphical Abstract
Keywords
INTRODUCTION
Worldwide textile, paper, and dye industries release their effluents containing organic dyes, in freshwater. The dyes are non-biodegradable, highly poisonous, containing colored pigments that are harmful to living organisms [1]. Numerous remedial plans are developed for the decontamination purpose of wastewater and some of them are related to the consumption of energy [2]. A photocatalyst is a material that absorbs solar light which is a renewable energy source. In recent years semiconductor material as a photocatalyst has attracted enormous attention for energy storage and in environmental fields [3-4]. It has great potential to solve energy and pollution-related issues [5-7].
Cu2O is a p-type semiconductor with a direct band gap of ~2.1 eV [8]. It is widely used as a photocatalyst due to its large abundance, non-toxicity, and easy preparation [9,10]. The attainment of semiconductor performance strongly depends on its structural factors viz., shape, size, and crystallization [11-14]. In the last few years, the shape and size-controlled Cu2O was synthesized in the form of various polymorphs like nanocubes [15], octahedra [16], nanowires [17], and cuboctahedra [18]. In addition to structural factors, the surface potential and electronic properties of the faces (110), (111), and (100) of Cu2O nanostructures also have a positive impact on photocatalytic performance. These properties were analyzed by using high-resolution Kelvin probe force microscopy [19] and it showed the surface energy order as 110>111>100 [20]. Among these (110) is a higher surface energy facet with a greater density of Cu swinging bonds on the surface. In recent studies, it was suggested that the nanostructures with higher energy facets exposed were more active and two different facets, with slight differences in surface energy, could accelerate the separation of photoexcited electron-hole pairs [21]. So, shape-controlled heterostructures play an important role in boosting photocatalytic performance. Therefore, the synthesis of material with exposed facets is an effective strategy to enhance the photocatalytic activity of Cu2O. Several research groups synthesized Cu2O with exposed 110 facets but they used a rigorous synthesis process and formed a less stable catalyst. To get rid of this problem, Su and coauthors developed a new process of Cu2O polyhedron synthesis [22].
Cuprous oxide has great potential in photocatalysis but it is suffering from photo corrosion induced by photo-reduction or photo-oxidation [23]. So, there is a need to synthesize a composite of Cu2O with other semiconducting materials. The inner electrostatic field of p-n heterojunction enhances the separation efficiency of electron-hole pairs thereby increasing photocatalytic performance [24]. There are many Cu2O semiconductor heterojunctions were reported such as WO3/Cu2O [25], Cu2O /ZnO [26], and CuO / Cu2O [27] having good to excellent degradation efficiency.
TiO2 is one of the most effective n-type semiconductors with a band gap of 3.2 eV [28]. Since it is a cheap material and has high chemical stability and high charge mobility [29-31], it is widely used as a good photocatalyst. However, it has some limitations such as low absorption of solar light and fast recombination rate of electron-hole pairs [32]. Generation of p-n heterojunction would be a good solution to exceed the limitations. Also, the p-n heterojunction enhances photocatalytic performance due to suitable alignments of band gaps at the interface of the p-n junction [33]. There are many TiO2 semiconductor heterojunctions were reported such as Nb2O5/TiO2 [34], CdS/TiO2 [35], and g-C3N4/TiO2 [36] with their excellent activity. Recently, many authors reported the synthesis of Cu2O/TiO2 [37, 38]. Table 1. shows the Cu2O and TiO2-based heterojunctions and their photocatalytic potential.
In the present study, we have synthesized a Cu2O/TiO2-QD photocatalyst for the degradation of Congo Red dye [39, 40]. UV-DRS, XRD, SEM, and TEM techniques were used to characterize the synthesized material. The photocatalytic performance of Cu2O/TiO2-QD was examined for the rapid decolorization of Congo red dye under visible light exposure at room temperature. Also, the degradation performance of Congo red dye at various concentrations and pH was analyzed using a UV-VIS spectrophotometer. The stability of a photocatalyst was studied by the XRD technique after use.
We have developed a simple and effective method for the synthesis of truncated octahedron and octahedron-shaped Cu2O by varying the amount of sodium hydroxide (0.1M).
EXPERIMENTAL
Reagents and Materials
Commercially available Copper chloride (CuCl2, Merck, 99.00%), Sodium hydroxide (NaOH, Loba, 99.00%), Ti-nanoxide R/SP (TiO2-QD, Sigma Aldrich, 99.00 %), Sodium dodecyl sulfate (SDS, Sigma Aldrich, 99.50%) and Congo red dye (CR, Sigma Aldrich, 99.00 %) were procured from Omkar Traders, Maharashtra, India and used without further purification. The stock solution and solution of the respective concentrations of Congo red dye (2, 4, 6, 8, 9, 10, and 15 mg/L) were prepared using deionized water.
Preparation of Cu2O
The copper (I) oxide nanoparticles were synthesized using the surfactant-assisted precipitation method. Herein, 7.5 ml, 0.1M Copper Chloride [CuCl2] was taken in a round bottom flask containing 100 mL deionized water, and 1.3 g Sodium dodecyl sulfate [SDS] was added to the above solution. To obtain a precipitate solution Cu(OH)2 with a pH of 8-9, 1M NaOH solution was slowly added. The variation in the amount of NaOH used is shown in Table 2. Then after 0.1 M NH2OH. The HCl solution was added under vigorous stirring and a color change was observed from blue to orange after the complete addition. The solution was kept in a water bath for 1 h and centrifuged at 5000 rpm for 5 min. The obtained precipitate was washed with enough water/ethanol (1:1) to remove the surfactant and dried at room temperature to obtain copper (I) oxide nanoparticles [20].
Synthesis of Cu2O/TiO2-QD Composite
10 mg copper (I) oxide nanoparticles in 10 ml ethanol were stirred for 30 min on a magnetic stirrer and commercially available TiO2-QD sol was added to it to obtain the precipitate. The mixture was centrifuged at 8000 rpm and the obtained precipitate was filtered and washed several times using ethanol: water (1:1) solvent. To get Cu2O/TiO2-QD composite the obtained precipitate was dried at room temperature.
MATERIAL CHARACTERIZATION
Different analytical techniques were used to characterize the synthesized material. The optical and band gap energy was studied by using UV-DRS analysis and it was performed on UV-DRS Shimadzu-1800. An X-ray diffraction analysis was carried out using a copper X-ray diffractometer (Cu Kα =0.1541 nm) operated at 40 kV and 40 mA. The morphology of the composite was analyzed using a Scanning Electron Microscope and Transmission Electron Microscope (Zeiss Libra 120).
Photocatalytic degradation experiments
The photocatalytic performance of the Cu2O/TiO2-QD composite was studied for the degradation of Congo red dye under visible light irradiation. The progress of the dye degradation was monitored by measuring the absorbance at regular time intervals using a UV-Vis spectrophotometer (Shimadzu-1800, India) at 498 nm. It was observed that the absorbance of the dye solution decreases with the increasing time of exposure, which indicates that the concentration of Congo red dye decreases. To estimate the degradation percentage of the photocatalyst following equation was used;
Where, C0 is the dye concentration at time zero, and Ct is the dye concentration at different times t.
RESULTS AND DISCUSSION
XRD Analysis
Figs. 2 (a) and 2(b) show the X-ray Diffractogram of Cuprous Oxide for cases (I) and (II) respectively. The sharp peaks at 2θ; 29.553, 36.417, 42.295, 52.452, 61.341, 69.566, 73.523, 77.320 corresponds to Miller indices (110), (111), (200), (211), (220), (310), (311) and (222) respectively, indicating the face-cantered cubic structure (JCPDS No. 05-0667). This Cu2O crystal has a cubic crystal structure in which an oxygen atom is present at the center with Cu atoms occupying half of the tetrahedral sites. No diffraction peaks of Cu or CuO were observed which indicates the high purity of synthesized material. Furthermore, the intensity ratio of the (200) and (111) peaks play an important role in differentiating their shapes and it was reported that the value of this ratio is lowered for truncated octahedron than octahedron shape [47] and our results reveal that the shape of particles in case (I) were of truncated octahedron shape and in case (II) corresponds to octahedron structure respectively. Also, the high intensity of peaks in XRD confirms the good crystallinity of particles. Fig. 2 (c) is the XRD pattern of TiO2-QD and it exhibits the anatase phase (JCPDS No. 21-1272). The broad diffraction peaks in XRD were observed due to the small crystallite size of TiO2-QDs. Fig. 2 (d) shows the XRD of Cu2O, TiO2, and Cu2O/TiO2-QDs composite. In the Cu2O/TiO2-QD composite, no characteristic peak of anatase-TiO2 was detected due to its low content and high dispersion in the composite.
The crystallite size of synthesized materials was calculated from XRD data using the Scherrer equation. The Scherrer equation can be written as:
Where, λ = 1.5406 Ả is the wavelength of Cu Kα radiations, k=0.9 is the shape factor, θ, and β are Bragg’s angle and FWHM respectively corresponding to the highly intense peak [48].
The calculated sizes of Cu2O, TiO2-QD, and Cu2O/TiO2-QD were found to be 60 nm, 12 nm, and 58 nm respectively. The result showed that the crystallite size of Cu2O and Cu2O/TiO2-QD are almost similar as there is a dispersion of TiO2-QD on the facets of Cu2O and it does not alter the shape and size of Cu2O.
SEM AND TEM analysis
The morphology and microstructure of Cu2O were confirmed using the SEM technique. Fig. 3. shows SEM images of Cu2O particles showing an average size of 0.5 µm and confirmed the truncated octahedron shape possessing 12 hexagonal (110) faces and 6 square (100) faces [49]. No other morphologies were detected in the SEM images which established the formation of mono-dispersed Cu2O with shape-controlled morphology. The hollow truncated octahedron shape case (I) of Cu2O was also depicted by the TEM technique and it is shown in Fig. 4(a), these results are impeccable with SEM analysis (Fig. 3(b) and 3(c)).
Fig. 4(b, c) shows TEM images of Cu2O (octahedron shape); case (II). The Cu2O/TiO2-QD composite is formed by loading TiO2 nanoislands on copper oxide nanoparticles. Fig. 4(d, e) TEM images somehow demonstrate that Cu2O/TiO2-QD heterojunctions were successfully generated.
Uv-visible diffused Reflectance Spectra
The optical properties of the samples were investigated by UV-visible diffused reflectance spectra. Fig. 5, shows the diffuse reflectance spectra of samples of pure TiO2, Cu2O, and Cu2O/TiO2-QD composites. Pure TiO2 showed absorption only in the UV region, while pure Cu2O showed strong absorption in the visible region. Cu2O/TiO2-QD composite is not only showing strong absorption in the UV region but also the absorption was shifted to the visible region when the amount of Cu2O increased. The composite showed greater absorbance, which may be because of good interaction between TiO2 and Cu2O, generating more stable hollow electron pairs thereby increasing photocatalytic performance.
Photocatalytic Study
Effect of pH
The effect of the pH of an aqueous solution on the CR dye was examined at different pH (4, 5, 6, 7, and 8) at 25˚C wherein the initial concentration of catalyst (150 mg/L) and concentration of CR dye (9mg/L) were taken. Fig. 6. showed that the degradation efficiency was increased from pH 4 to 6 whereas it was decreased at pH 7 and 8. The pH affects the surface charge of the photocatalyst. Every photocatalyst has a property of pHzpc (50). The photocatalytic surface will become positively charged at low pH than pHzpc which adsorb anionic molecules preferentially. pH beyond pHzpc of photocatalyst, results in negatively charged surface and adsorb cationic dye molecules (51). As CR dye is an anionic diazo dye it will show more photocatalytic efficiency at pH 6.
Effect of initial concentration of CR on effluent
The effect of various concentrations (2, 4, 6, 8, 9, 10, and 15 mg/L) of Congo red dye was studied at 25 ˚C and pH =6, wherein the initial concentration of catalyst was 150 mg/L. Fig. 7. revealed that the percentage degradation of the CR dye increases initially with an increase in the concentration of dye up to 9 mg/L. It is because of the availability of more adsorption sites on the photocatalyst surface which enhances the ability of adsorption of the CR molecules. These sites are saturated with time as they get covered with the adsorbed molecules causing a decline in the percentage of photodegradation with a further increase in concentration [52].
Effect of Cu2O/TiO2-QD dosage
The effect of Cu2O/TiO2-QD dose on the CR dye was examined using various catalyst concentrations (100, 150, 200 mg/L) at 25 ˚C and the initial concentration of CR dye was 9mg/L. Fig. 8. Shows that the degradation efficiency for the concentration 150 mg/L of Cu2O/TiO2-QD was greater than 100mg/L and 200mg/L concentrations. In general, the rate of photocatalytic degradation of organic pollutants increases with photocatalyst dose, due to an increase in active sites. But if the concentration of the catalyst increases beyond the optimum amount the scattering effect of light also increases thereby decreasing degradation efficiency [53]. So, in the present study, 150 mg/L of Cu2O/TiO2-QD catalyst concentration was selected as the optimum concentration for CR dye degradation.
Reusability of the photocatalyst
To prove the excellent efficiency of a prepared photocatalyst, the reusability of the photocatalyst was examined by performing recycling experiments. After the recycling experiment, the structural stability of the recycled photocatalyst was confirmed from XRD analysis wherein no change in XRD peak positions was observed (Fig. 9). It reveals the good stability of the photocatalyst.
Photocatalytic Degradation of CR dye at optimum conditions
The present work deals with the photocatalytic study of shape-controlled Cu2O/TiO2-QD composite. The photocatalytic experiments were conducted by exposing the catalyst and dye solution to the visible light source. Fig. 10 shows the degradation results of Congo red dye. A comparison between the photocatalytic performance of as-synthesized Cu2O/TiO2-QD composite and reported heterojunction revealed that the shape-controlled Cu2O/TiO2-QD composite has higher efficiency and capability of dye degradation [54-56].
Photocatalysis mechanism
Fig. 11. indicates a schematic view of the charge transfer processes of a prepared photocatalyst under visible light irradiation. When visible light incident on the photocatalyst, the electrons-hole pairs get produced in which the photogenerated electrons in the conduction band transfer to the conduction band of TiO2, and at the same time hole on the valence band of TiO2 transfers to the valence band of Cu2O [57]. Hence, the recombination rate of electron-hole pairs decreases, and the OH. and O2.- radicals carry out further degradation reactions. The proposed mechanism of degradation of CR dye is given in Fig. 11.
CONCLUSION
In this work, a simple precipitation method was used to synthesize truncated octahedron and octahedron Cu2O and Cu2O/TiO2-QD composite. The morphological, structural, and optical properties of the as-prepared material were investigated by XRD, SEM, TEM, and UV-visible DRS techniques. TEM images clearly show that the TiO2 was highly dispersed and firmly anchored on single crystals of copper oxide in Cu2O/TiO2-QD composite. The photocatalytic efficiency was investigated by studying factors like the influence of pH, catalyst dose, the concentration of dye, and reusability of the photocatalyst and it was found that 89% of Congo red dye degradation occurred at pH=6 within 110 minutes using 150 mg/L of catalyst concentration. For the first time, we have reported the greater degradation (89.00%) of Congo red dye by Cu2O/TiO2-QD photocatalyst. Experimental findings showed that Cu2O/TiO2-QD composite has a substantial ability to degrade CR dye, and its usage in the treatment of effluents containing this dye is suggested.
CONFLICT OF INTEREST
The authors hereby declare that there is no conflict of interest.
Abbreviations and symbols
|
SDS |
Sodium dodecyl sulphate |
|
UV-DRS |
Ultra-Violet diffuse reflectance spectroscopy |
|
XRD |
X-Ray Diffraction |
|
SEM |
Scanning Electron Microscopy |
|
TEM |
Transmission Electron Microscopy |
|
QD |
Quantum Dots |
|
CR |
Congo red |
|
Cu2O |
Copper oxide |
|
Cu |
Copper |
|
CdS |
Cadmium sulfide |
|
WO3 |
Tungsten oxide |
|
Fe2O3: |
ferric oxide |
|
TiO2: |
titanium oxide |
|
CuCl2: |
Copper chloride; |
|
NaOH: |
Sodium hydroxide; |
|
e - |
Electron |
|
h + |
Hole |
|
hν |
Energy (photon |
|
λ |
Wavelength |
|
β |
Peak broadness at full width of half maximum intensity |
|
2θ |
Bragg diffraction angle |
|
nm |
Nanometres |
- Travis, A., 1997. Poisoned groundwater and contaminated soil: the tribulations and trial of the first major manufacturer of aniline dyes in Basel. Environmental History, 2(3):343-365.
https://doi.org/10.2307/3985354 - Aravind, P., H. Selvaraj, S. Ferro, M. Sundaram, 2016. An integrated (electro-and bio-oxidation) approach for remediation of industrial wastewater containing azo-dyes: understanding the degradation mechanism and toxicity assessment. Journal of hazardous materials, 318: 203-215. https://doi.org/10.1016/j.jhazmat.2016.07.028
- Zhang, F., X. Wang, H. Liu, C. Liu, Y. Wan, Y. Long, Z. Cai, 2019. Recent Advances and Applications of Semiconductor Photocatalytic Technology. Applied Sciences, 9(12): 2489. https://doi.org/10.3390/app9122489
- Hochbaum, A., P. Yang, 2010. Semiconductor Nanowires for Energy Conversion. Chemical Reviews, 110(1): 527-546. https://doi.org/10.1021/cr900075
- Shaterian, M., H. Ardeshiri, R. Mohammadi, Z. Aghasadeghi, M. Karami, 2023. Synthesis, characterization, and electrochemical evaluation of SnFe2O4@MWCNTS nanocomposite as a potential hydrogen storage material. Heliyon, 9(6): 16648. https://doi.org/10.1016/j.heliyon.2023.e16648
- Shaterian, M., M. Yulchikhani, Z. Aghasadeghi, H. Ardeshiri, 2022. Synthesis, characterization, and investigation of electrochemical hydrogen storage capacity in barium hexaferrite nanocomposite. Journal of Alloys and Compounds, 915:165350. https://doi.org/10.1016/j.jallcom.2022.165350
- Juntadech, N. T. Juntadech, S. Niamlang, B. Jongsomjit, 2023. Cellulose acetate/Nano clay composite membranes with enhanced mechanical properties and improved permeance in gas separation. Journal of Applied Polymer Science, 54032. https://doi.org/10.1002/app.54032
- Bagal, I., N. Chodankar, M. Hassan, A. Waseem, M. Johar, D. Kim, 2019. Cu 2 O as anEmerging Photocathode for Solar Water Splitting - A Status Review. International Journal of Hydrogen Energy, 44(39): 21351-21378. http://dx.doi.org/10.1016/j.ijhydene.2019.06.184
- Ali, S., A. Razzaq, H. Kim, 2021. Activity, Selectivity, and Stability of Earth-Abundant CuO/Cu 2 O/CuO-based Photocatalysts Toward CO 2 Reduction. Chemical Engineering Journal, 422022-131579. http://dx.doi.org/10.1016/j.cej.2021.131579
- Bhosale, M., M. Bhanage, 2016.A simple approach for sonochemical synthesis of Cu 2 O nanoparticles with high catalytic properties, Advanced Powder Technology, 27(1) 238-244. http://dx.doi.org/10.1016/j.apt.2015.12.008
- Zhou, T., T. Zhan, J. Deng, R. Zhang, L. Zheng, L. Wang, 2017. P-type Co3O 4Nanomaterials-based Gas Sensor: Preparation and Acetone Sensing Performance. Sensors and Actuators B-chemical, 242:369-377. http://dx.doi.org/10.1016/j.matpr.2020.04.857
- Xia, Y., Y. Xiong, B. Lim, S. Skrabalak, 2009. Shape-Controlled Synthesis of MetalNanocrystals: Simple Chemistry Meets Complex Physics. Angewandte Chemie International Edition, 48: 60-103. https://doi.org/10.1002/anie.200802248
- Kéri, O., E.Kocsis, D.A.Karajz, Z.K.Nagy, B.Parditka, Z.Erdélyi, A.Szabó, K.Hernádi, I.M. Szilágyi, 2021. Photocatalytic Crystalline and Amorphous TiO 2 Nanotubes Prepared by Electrospinning and Atomic Layer Deposition. Molecules, 26(19):5917. https://doi.org/10.3390/molecules26195917
- Sayed, M., 2004. Small Is Different: Shape-, Size-, and Composition-Dependent Properties of Some Colloidal Semiconductor Nanocrystals. Accounts of Chemical Research, 37(5): 326-333. https://doi.org/10.1021/ar020204f
- Kuo, C., M. Chen, H. Huang, 2007. Seed-Mediated Synthesis of Monodispersed Cu 2 O Nanocubes with Five Different ranges from 40 to 420 nm. Advanced Functional Materials, 17(18): 3773-3780. http://dx.doi.org/10.1002/adfm.200700356
- Haolan, X., W. Wenzhong, Z. Wei,2006. Shape Evolution and Size-Controllable Synthesis of Cu2O Octahedra and Their Morphology-Dependent Photocatalytic Properties. The Journal of Physical Chemistry B,110(28):13829-13834. https://doi.org/10.1021/jp061934y
- Das, K., S. De, 2009. Optical and Photoconductivity Studies of Cu 2 O Nanowires Synthesized by Solvothermal Method. Journal of Luminescence, 129:1015-1022.
https://doi.org/10.1016/j.jlumin.2009.04.019 - Zhang, D., H. Zhang, L. Guo, K. Zheng, Z. Zhang,2009. Delicate Control of Crystallographic Facet-Oriented Cu 2 O Nanocrystals and the Correlated Adsorption Ability. Journal of Materials Chemistry. Mater, 19(29):5220-5225. https://doi.org/10.1039/B816349A
- Lee, S., W. Liang, W. Martin, 2011.Synthesis, Control, and Characterization of Surface Properties of Cu 2 O Nanostructures. ACS Nano, 5:3736-3743. https://doi.org/10.1021/nn2001933
- Xu, X., Z. Gao, Z. Cui, Y.Liang, 2016. Synthesis of Cu 2 O Octa decahedron/TiO 2 Quantum Dot Heterojunctions with High Visible Light Photocatalytic Activity and High Stability. ACS Applied Materials Interfaces, 8(1):91-101. http://dx.doi.org/10.1021/acsami.5b06536
- Huang, M., P. Lin,2012. Shape-controlled synthesis of polyhedral nanocrystals and their facet-dependent properties. Advanced Functional Materials, 22(1):14-24. https://doi.org/10.1002/adfm.201101784
- Su, Y., H. Li, H. Ma, Robertson, A. Nathan, 2017. Controlling Surface Termination and Facet Orientation in Cu 2 O Nanoparticles for High Photocatalytic Activity: A Combined Experimental and Density Functional Theory Study. ACS Applied Materials and Interfaces, 9(9):8100-8106. https://doi.org/10.1021/acsami.6b15648
- Cui, Y., S. Jason, A.Rose, H. Yun, 2019. Recent advances in suppressing the photocorrosion of cuprous oxide for photocatalytic and photoelectrochemical energy conversion. Journal of Photochemistry and Photobiology C: Photochemistry Reviews, 40:191-211. https://doi.org/10.1016/j.jphotochemrev.2018.10.001
- Qiujian, X., Y.Yang,Yumin & Zhang, Weijie & Gao, Zhu & Li, Xiaofeng & Tang, Juntao &Pan, Chun-yue & Yu, Guipeng, 2021. Polarization-Induced Charge Separation in Conjugated Microporous Polymers for Efficient Visible Light-Driven C-3 Selenocyanation of Indoles. Chemical Science,12. https://doi.org/10.1039/D0SC06951E
- Zhang, J., H. Ma, Z. Liu, 2017. Highly efficient photocatalyst based on all oxides WO3/Cu2O heterojunction for photoelectrochemical water splitting. Applied Catalysis B: Environmental, 201:84-91. https://doi.org/10.1016/j.apcatb.2016.08.025
- Yang, M., L. Zhu, Y. Li, L. Cao, Y. Guo, 2013. Asymmetric interface band alignments of Cu2O/ZnO and ZnO/Cu2O heterojunctions. Journal of alloys and compounds, 578: 143-147. https://doi.org/10.1016/j.jallcom.2013.05.033
- Wijesundera, R., 2010. Fabrication of the CuO/Cu2O heterojunction using an electrodeposition technique for solar cell applications. Semiconductor science and technology, 25(4): 045015. https://doi.org/10.1088/0268-1242/25/4/045015
- Nakata, K., A. Fujishima, 2012. TiO 2 photocatalysis: Design and Applications. Journal of photochemistry and photobiology C: Photochemistry Reviews, 13(3):169-189. https://doi.org/10.1016/j.jphotochemrev.2012.06.001
- Chen, X., S.Mao, 2007. Titanium Dioxide Nanomaterials: Synthesis, Properties,Modifications, and Applications. Chemical reviews, 107(7):2891-2959. https://doi.org/10.1021/cr0500535
- Brittain, H., G. Barbera, 1992. Titanium Dioxide. Analytical Profiles of Drug Substances and Excipients. 19:659-691. https://doi.org/10.1016/S0099-5428(08)60404-9
- Bandar, A., H. Mohammad, Y. Chi Chin, M. Muhamad, 2013. Influence of TiO2 Nanoparticles on Enhancement of Optoelectronic Properties of PFO-Based Light Emitting Diode. Journal of Nanomaterials, 7:561534. https://doi.org/10.1155/2013/561534
- Ranjit, K., B. Viswanathan, 1997. Synthesis, Characterization and Photocatalytic Properties of Iron-Doped TiO2 Catalysts. Journal of Photochemistry and Photobiology A. Chemistry, 108(1):79-84. https://doi.org/10.1016/S1010-6030%2897%2900005-1
- Paramanik, L., K. Reddy, K. Parida, 2019. Photocatalytic Activity of p-BiOI/n-PbTiO 3 Heterojunction: The Significant Role of Oxygen Vacancies and Interface Coupling. The Journal of Physical Chemistry C,123(35):21593-21606. http://dx.doi.org/10.1021/acs.jpcc.9b05747
- Yan, J., G. Wu, N. Guan, L. Li, 2014. Nb2O5/TiO2 heterojunctions: synthesis strategy and photocatalytic activity. Applied Catalysis B: Environmental, 152:280-288. https://doi.org/10.1016/j.apcatb.2014.01.049
- Bessekhouad, Y., D. Robert, J. Weber, 2004. Bi2S3/TiO2 and CdS/TiO2 heterojunctions as an available configuration for photocatalytic degradation of organic pollutant. Journal of Photochemistry and Photobiology A: Chemistry, 163(3): 569-580. https://doi.org/10.1016/j.jphotochem.2004.02.006
- Alcudia-Ramos, A., M. Fuentez-Torres, F. Ortiz-Chi, G. Espinosa-González, N. Hernández‐Como, S. García-Zaleta, S. Godavarthi, 2020. Fabrication of g-C3N4/TiO2 heterojunction composite for enhanced photocatalytic hydrogen production. CeramicsInternational, 46(1):38-45. https://doi.org/10.1016/j.ceramint.2019.08.228
- Lee, K., H. Yoon, C. Ahn, J. Park, S. Jeon, 2019. Strategies to improve the photocatalytic activity of TiO2: 3D Nanostructuring and Heterostructuring with Graphitic Carbon Nanomaterials. Nanoscale, 11(15):7025-7040. https://doi.org/10.1039/C9NR01260E
- Yan, X., M.Wang, Y.Xiaojie, 2013. Band Structure Design of Semiconductors for Enhanced Photocatalytic Activity: The Case of TiO2. Progress in Natural Science: Materials International, 23(4):402-407. https://doi.org/10.1016/j.pnsc.2013.06.002
- Sakkas, V., I. Azharul, C. Stalikas, A. Triantafyllos, 2010. Photocatalytic degradation using design of experiments: A review and example of the Congo red degradation. Journal of Hazardous Materials, 175(1-3):33-44. http://dx.doi.org/10.1016/j.jhazmat.2009.10.050
- Chedly,C., A. Nedra, A. Lamia, H. Neila, H. Moktar, 2018. Congo Red Decolorization and Detoxification by Aspergillus niger: Removal Mechanisms and Dye Degradation Pathway.Biomed research International, 3049686. https://doi.org/10.1155/2018/3049686
- Yingying, S., Y. Zewei, W. Bing, A. Hao, C. Zezhi, C. Hao, 2016. Adsorption and photocatalytic degradation of tetracycline hydrochloride using a palygorskite-supported Cu2O-TiO2 composite. Applied Clay Science, 119(2):311-320. https://doi.org/10.1016/j.clay.2015.10.033.
- Xiaojiao, Yu., Z. Chen, L. Zongbin, W. Yuchen, Y. Meng, G. Biqi, N. Jinfen, 2023. Preparation of ZnO/Cu2O Composite Particles and Its Degradation of Ciprofloxacin: Analysis of Degradation Performance and Active Species. Integrated Ferroelectrics, 2 36(1):96-108. https://doi.org/10.1016/j.clay.2015.10.033.
- Abbas, N., N. Alireza, 2020. α-Fe2O3/Cu2O heterostructure: Brief characterization and kinetic aspect of degradation of methylene blue. Physica B: Condensed Matter, 599:412422. https://doi.org/10.1016/j.physb.2020.412422
- Ajmal, A., I. Majeed, R. Malik, M. Iqbal, M. Nadeem, I. Hussain, S. Yousaf, G. Mustafa, M. Zafar, 2016. Photocatalytic degradation of textile dyes on Cu2O-CuO/TiO2 anatase powders. Journal of Environmental Chemical Engineering, 4(2): 2138-2146, https://doi.org/10.1016/j.jece.2016.03.041
- Shokri, A., K. Mahanpoor, 2016. Removal of ortho-toluidine from industrial wastewater by UV/TiO2 process. 213-223. https://jchr.damghan.iau.ir/article_544149.html
- Shokri, A., 2022. Photocatalytic degradation of nitrotoluene in synthetic wastewater by CoFe2O4/SiO2/TiO2 nanoparticles using Box-Behnken experimental design, Desalination and Water Treatment. 247:92-99.
https://doi.org/10.5004/dwt.2022.28037 - Bhaisare.m., M.Khan, S.Pandey, G.Gedda, 2017. Shape Oriented Photodynamic Therapy of Cuprous Oxide Nanocrystals for Cancer Treatment. RSC Advances, 7:23607-23614. https://doi.org/10.1039/C6RA28705K
- Cullity, B., 1956. Elements of X-ray diffraction. Reading, Mass.: Addison-Wesley Pub. Co.
- Yang, X., J. Li, J.Yao ,T. Ren , B.Zhang, 2021. Hydroxyl ions: flexible tailoring of Cu2O crystal morphology. RSC Advances, 11(60): 37760-37766. https://doi.org/10.1039/D1RA03296H
- Shokri, A., T. Abmatin, 2017. Employing photo fenton and UV/ZnO processes for removing reactive Red-195 from aquous environment. Fresen Environ Bull. 26, 1560-1565. https://hero.epa.gov/hero/index.cfm/reference/details/reference_id/4400692
- Kalantar, K., S. Gnanaprakasam, V.Thirumarimurugan, 2015. Influencing Parameters in the Photocatalytic Degradation of Organi Effluent via Nanometal Oxide Catalyst: A Review Indian Journal of Materials Science, 601827. https://doi.org/10.1155/2015/601827
- Mohammed, A., S. Mohtar, F. Aziz, M. Aziz, A. Ul-Hamid, 2021.Cu 2 O/ZnO-PANI ternary nanocomposite as an efficient photocatalyst for the photodegradation of Congo Red dye.Journal of Environmental Chemical Engineering, 9(2):105065. https://doi.org/10.1016/j.jece.2021.105065
- Crini, G.,2006. Non-conventional low-cost adsorbents for dye removal: a review. Bioresour Technol. 97(9):1061-85. https://doi.org/10.1016/j.biortech.2005.05.001
- Sadik, W., A. El-Demerdash, A. Nashed, A. Mostafa, H. Hamad, 2019. Highly efficient photocatalytic performance of Cu 2 O@TiO 2 nanocomposite: influence of various inorganic oxidants and inorganic anions. Journal of Materials Research and Technology, 8(6): 5405- 5414. https://doi.org/10.1016/j.jmrt.2019.09.007
- Donawade, D., A. Raghu, G. Gadaginamath, 2006. Synthesis and antimicrobial activity of sum new 1-substituted-3- pyrroryl aminocarbonyl/ oxadiazolyl/triazolyl/ 5-methoxy-2-methylindoles and benz[g]indoles. Indian Journal of Chemistry, 45B, 689-696. https://doi.org/10.1002/chin.200628116
- Donawade, D., A. Raghu, G. Gadaginamath, 2005. Chemoselective reaction of benz(g)indole based bisheterocycle dicarboxylate towards hydrazine hydrate:synthesis and antimicrobial activity of new triheterocycles-5-pyrolylaaminocarbonyl/mercaptooxadiazolyl/4-allyl-5-mercaptotriazolylmethoxy-1-furfuryl-2-methylbenz(g)indoles. Indian Journal of Chemistry, 44B, 1470-1475. https://doi.org/10.1002/chin.200545144
- Hosseinzadeh, G., 2023. Synthesis of novel p-n heterojunction photocatalyst from ZnO nanorod and Cu2O nanoparticles for degradation of Paraoxon insecticide under visible light irradiation, Journal of Water and Environmental Nanotechnology, 8(10):13-22. https://doi.org/10.22090/jwent.2023.08.002