Document Type : Original Research Paper
Authors
1 Faculty of Chemical and Petroleum Engineering, University of Mohaghegh Ardabili, Ardabil
2 Faculty of Chemical and Petroleum Engineering, University of Tabriz, Tabriz
3 Department of Environmental Engineering, College of Engineering, Mustansiriyah University, Baghdad, Iraq
Abstract
This study investigated the removal of methylene blue dye from aqueous solutions using Mg solid state exchanged bentonite. Parent bentonite and magnesium exchanged bentonite were characterized by scanning electron microscopy (SEM), X-ray diffraction (XRD), Brunauer Emmett Teller (BET), Energy dispersive X-ray spectroscopy (EDX), and Fourier transform infrared spectroscopy (FTIR). The parent bentonite showed the lowest removal efficiency (40.3%) and prepared magnesium exchanged bentonite for 10 min showed the highest removal efficiency (54.8%). Thus, dye absorption by modified bentonite increased compared to parent bentonite. After its modification by the ion exchange method, the specific surface was changed from 44.5 to 56.05 M2/g. EDX results indicate that the cation exchange process has been completed successfully. Ion exchange causes some minor changes in bentonite morphology and crystallinity. The best conditions for removing methylene blue using magnesium exchanged bentonite was pH 10, adsorbent dosage 0.05 mg/liter, initial concentration of solution 100 mg per liter, and contact time of 120 minutes with a Removal efficiency of 96.67. The Langmuir isotherm had a better correlation coefficient than the Freundlich adsorption model, which indicated the homogeneous surface of the adsorbent.
Graphical Abstract
Keywords
Introduction
Wastewater from the textile industry can be problematic due to the presence of hazardous waste and toxic pollutants. Meanwhile, organic dyes are one of the main groups of pollutants [1]. The release of toxic and dangerous dyes present in textile effluents has created global issues due to their considerable toxicity. Dyes make the water an unpleasant color and are fatal for aquatic life [2]. In addition, modern dyes are chemically, photolytically, and microbiologically stable. Therefore, dyes cannot be easily degraded during conventional biological aerobic treatment [3]. Until now, various methods such as coagulation, chemical decomposition, membrane separation, electrochemical methods, and adsorption techniques have been used to remove pollutants [4-6]. Compared to the mentioned methods, physical adsorption is generally considered an effective method due to its high efficiency, simplicity, variety of adsorbents, and lower cost [1, 7-9].
Extensive studies on the adsorption of dyes by various adsorbents including activated carbon, silica, clay, natural and synthetic polymers, waste materials, various nanomaterials, alumina, etc. have been reported [10-13]. Meanwhile, metal oxides seem to have higher adsorption efficiency [14, 15]. In this context, magnesium oxide nanoparticles attractively showed a high adsorption capacity [16, 17]. In addition, these nanoparticles have low solubility in water, high specific surface area, high alkalinity and high PZC, wide band gap, high thermodynamic stability, low dielectric constant, and low refractive index. As a result, these nanoparticles can be used to treat polluted water with minimal harmful effects on the environment [18,19].
However, due to the high surface energy, nanoparticles tend to form agglomerates [19]. This greatly reduces their specific surface area and activities and reduces their adsorption and catalytic performance. Immobilization of nanoparticles on a suitable substrate can be an effective solution to overcome these shortcomings [10]. Among the different types of substrates, clays can be classified as hydrated aluminosilicates [20]. Bentonite is clay with a 2:1 layer structure in which an octahedral sheet is placed between two tetrahedral silica sheets. The surface charge of this material is negative, which helps to maintain cationic ions. Bentonite samples can remove cationic ions from solutions through electrostatic interactions and ion exchange reactions [21]. On the other hand, they repel anions due to their negative surface charge. This can be solved by modification techniques [22].
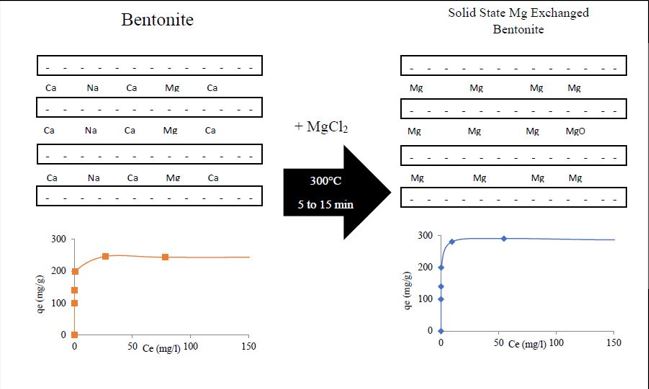
Incorporation of nanoparticles and clay can be done by various methods such as solvothermal, chemical vapor deposition, sol-gel, and chemical precipitation [23-26]. Each of these mentioned methods has its advantages and disadvantages. The simplest under-development method is the solid-state ion exchange method [27-29]. Compared to other methods, solid-state ion exchange is simpler, faster, and more cost-effective [30]. According to the published reports, the cation/clay composites prepared by the solid phase ion exchange method are very stable, so the amount of cation released in the aqueous solution is very small. In this method, clay is mixed with suitable magnesium salt and heated at a temperature close to the melting point of the salt. After melting, the salt flows and penetrates the pores of the clay, and ion exchange takes place. The chemical reaction is represented as follows:
In addition, heating in the air atmosphere causes the exchanged Mg2+ ions to convert to magnesium oxide particles. Oxidation of magnesium is done slowly in the presence of air at a temperature of 150°C. At a temperature of 300, this reaction occurs at a higher rate, so it is well-fitted by an inverse logarithmic law [31].
In the present research, 300°C has been used for ion exchange between bentonite and magnesium chloride. On the other hand, the dimensions of exchanged magnesium which inters the interlayer space of bentonite are in the atomic scale. So, easy and fast oxidation of magnesium can be expected.
According to our research, the adsorption properties of solid-state ion-exchanged bentonite with magnesium have not been studied yet. In this work, magnesium exchanged bentonite was considered to absorb dye from an aqueous solution. To reach the optimum conditions of the adsorption process, the effect of pH, adsorbent dosage, temperature, and contact time were investigated. In addition, the physicochemical properties of the adsorbents were investigated.
Materials and methods
Preparation of ion exchange bentonite
Magnesium chloride (2.5 g) was mixed with parent bentonite (5 g) in a crucible. After milling the magnesium chloride and its thoroughly mixing with bentonite, the sample was heated at 300°C for (5, 10, and 15) min. These samples were named 5, 10, and 15 respectively. Then, the sample was repeatedly sonicated and washed with distilled water to remove excess ions from the sample. Next, the material was settled and washing was done again. After washing, the adsorbent was filtered and placed in an oven at 50°C for 24 hr to dry completely.
Adsorption experiments
The prepared adsorbents (0.025 mg/l) were added into 50 ml methylene blue solution (100 mg/l) and stirred for 2 hr. Then, the suspension was centrifuged and the residual dye concentration in the supernatant solution was measured by a spectrophotometer. The dye removal efficiency (%) was determined as follows
Where C0 is the initial dye concentration (mg/l) and Ce is the equilibrium concentration of dye (mg/L). The equilibrium dye adsorption (mg/g) was determined as follows
Where m is the mass of adsorbent (g) and V is the volume of dye solution (L). This test was systemically carried out at various conditions including different adsorbent dosages (0.015 to 0/06 g/l), contact time (5, 10, 20, 30, 45, 60, 90, and 120) min., initial dye concentration (50, 75, 100, 150, 300, 200 and 400) mg/l, solution pH (between 5.5 and 10) to investigate the influence of various factors.
Adsorption isotherm
Langmuir isotherm could be arranged in its linear form as Eq. (5):
Where qm and KL are Langmuir constants relating to adsorption density (mg/m2) and the energy of adsorption (L/g), respectively. The adsorption data were also fitted to Freundlich isotherm, which is described by the linear form following Eq. (4):
Where KF and n are Freundlich constants incorporating all factors affecting the adsorption density and intensity of adsorption, respectively.
Characterization
The X-ray diffraction (XRD) patterns of the samples were characterized using an X-ray diffractometer (Philips PW 1050, The Netherlands) with CuKα radiation (λ = 1.5418 Å, 40 kV and 30 mA, 2θ from 0 to 80° and 0.05° step). Scanning electron microscopy (SEM) and Elemental dispersive X-ray spectroscopy (EDX) were carried out with an LEO 1430VP instrument. A Micromeritics BET surface area and porosity analyzer (Gemini 2375, Germany) was used to evaluate the products with N2 adsorption/desorption at the constant temperature of 77 K in the relative pressure range of 0.05-1.00.
Results
Characterization
BET analysis
Table. 1 presents the results of BET analysis for parent bentonite and ion exchange bentonite with magnesium. The specific surface area of the primary bentonite was 44.5 m2/g. After its modification by the ion exchange method, the specific surface was changed to 46.9, 56.05, and 38.8 m2/g for samples 5, 10, and 15, respectively. Fig. 1 presents the nitrogen adsorption-desorption isotherms for bentonite and optimum magnesium ion exchange bentonite adsorbent (sample 30). This figure shows that the behavior of both adsorbents follows the third type of isotherm.
SEM images
In Fig. 2, SEM images of parent bentonite and the optimum magnesium exchanged bentonite (sample 30) are presented. According to the obtained results, it can be seen that parent bentonite has an irregular, spongy appearance and layered structure (Figure 2. a). According to Figure 2-b, during ion exchange, the primary layered structure of bentonite is preserved, but some minor changes are observed. During the process of ion exchange, the layers have become slightly open. It seems that this is caused by the heating of bentonite at 300 ᵒC. In this state, magnesium can more easily penetrate the interlayer structure of bentonite. Thus, there are not any Mg-related particles on its surface. The obtained results are in agreement with the results of previous research that has been carried out on the ion exchange of bentonite with iron and copper [27, 28, 32].
EDX
Table. 2 shows the results of the EDX analysis of bentonite and optimum sample. According to the presented table, the existence of magnesium, potassium, and calcium in the interlayer structure of bentonite has been confirmed. The task of interlayer cations is to stack silicate layers and connect them. As can be seen, during the ion exchange process, calcium has been completely removed from bentonite layers. Also, the amount of potassium and iron has decreased and the amount of magnesium has increased. All these changes indicate that the cation exchange process has been completed successfully. Complete removal of calcium, short time, and increase of magnesium content in bentonite indicate the efficiency of this method over other methods.
XRD analysis
In Fig. 3, XRD spectra of parent bentonite and magnesium-exchanged bentonite samples are presented. The present peaks around the 2θ of 20.7ᵒ, 26.53ᵒ, and 49.9ᵒ indicate the presence of quartz impurity in bentonite structure. Also, the presence of calcite in bentonite is shown by the peak at about 27.9ᵒ [27,32]. The peak at 2θ =6.30º corresponds to the d001 basal spacing equal to 1.41 nm which is typical for calcium-rich montmorillonites. As shown in the figure, during the first 5 minutes of the ion exchange reaction, the basal spacing increased. This distance increased to 1.42 nm. In the sample ion exchange for 10 minutes, this distance was changed to 1.42 nm. But in the sample 15 min, it decreased to 1.41 nm due to the loss of water in the interlayer space [30]. Also, no peaks belonging to impurities were observed, which is due to the high purity of the modified samples.
FT-IR analysis
Fig. 4 shows the results of FT-IR analysis for parent bentonite and the optimum sample in the range of 400 cm-1 to 4000 cm-1. In the spectrum of bentonite, the vibrations related to the hydroxyl groups of water adsorbed by bentonite are represented by the peaks located at around 3632 cm-1 and 1658 cm-1. The 1048 cm-1 peak shows the stretching vibration of the Si-O bond. Also, the 952 cm-1 peak is related to the bending vibration of the Al–Al–OH bond. The sharp peak at 798 cm-1 and 736 cm-1 shows the presence of quartz in bentonite [33]. After the ion exchange of bentonite with magnesium chloride, all of the mentioned peaks were observed, although with slight changes in the intensity and position of the peaks. It seems that these changes are related to the ion exchange process. Finally, in exchanged bentonite samples, no peaks related to the Mg-O bond (490, 599, 1370, and 1580 cm-1) are observed. This can be caused by the overlapping of these peaks with bentonite peaks.
Adsorption experiments
The removal efficiency of methylene blue from the aqueous solution by the synthesized and bentonite samples is presented in Fig. 5. Sample (0) shows the removal efficiency of parent bentonite. Sample 15 showed the lowest removal efficiency (36.58%) and sample 10 showed the highest removal efficiency (54.8%) and it was identified as the optimum sample.
Based on the XRD and BET results, with the progress of the ion exchange process, the specific surface area of bentonite increases during the first 10 min. and then decreases with increasing time. Therefore, in the 5- and 10-min. samples, the increase in specific surface area leads to an increase in surface absorption. Further, with the reduction of the specific surface area, the surface absorption also decreases.
Effect of pH
Fig. 6 shows the effect of solution pH on the removal efficiency of methylene blue by the optimum sample of exchanged bentonite with magnesium. According to the graph, the lowest value for methylene blue adsorption percentage was detected at pH=5.5. This amount increased with the increase of pH up to 10, and at pH equal to 10, 96.67% of methylene blue was removed. With increasing pH, the negative charges of the exchanged bentonite surface made electrostatic attraction with cationic dye. But in acidic pHs, the surface is positively charged, making electrostatic repulsion with dye [34].
Effect of adsorbent dosage
The effect of the amount of adsorbent used on the Removal efficiency of methylene blue from the aqueous solution is presented in Fig. 7. Adsorbent concentration indicates the number of adsorption sites available to remove methylene blue from the aqueous solution. By keeping the parameters of time, concentration, and pH constant, and by increasing the adsorbent dosage, the number of active sites for methylene blue adsorption has been increased. With the change of adsorbent dosage from 0.015 g/l to 0.06 g/l, the color Removal efficiency increased with an upward trend. Therefore, the value of 0.05 g/l was reported as the optimum amount of adsorbent.
Effect of Initial Dye Concentration
Methylene blue solution was prepared with different concentrations by keeping the pH, time, and the adsorbent dosage constant at 10, 120 min., and 0.05 mg/l, respectively (Fig. 8). According to the figure (equilibrium concentration versus equilibrium adsorption), the equilibrium adsorption (qe) increases with the increase of methylene blue dye concentration. Increasing initial dye concentration led to an increase in the dye uptake onto adsorbents to a certain point; then, a plateau occurred for both adsorbents that indicated the unavailability of adsorption sites. Due to the saturation of the adsorbent at higher concentrations, the adsorption reaches its maximum value and remains constant.
Isotherm
The Langmuir and Freundlich equations are generally used to describe the adsorption isotherm, which explains the specific relation between the concentration and amount of adsorbate adsorb on the surface of the adsorbent. To compare the adsorption isotherm of parent and magnesium-exchanged bentonite, the experimental data fit Langmuir and Freundlich equations. Fig. 9 presents the obtained results. It can be seen that the Langmuir isotherm matches the results better than the Freundlich isotherm which is confirmed by the high value of R2 = 0.99 as compared to R2 = 0.83 for the Freundlich adsorption isotherm. This shows that the optimum absorbent surface is homogeneous and the available sites on the absorbent surface are uniformly distributed. Langmuir isotherm is mostly used to investigate chemical adsorptions in which a chemical bond is formed between the adsorbent and the adsorbed substance. It indicated monolayer uniform adsorption on the surface of the adsorbent [35]. The parameters obtained from Langmuir isotherms are reported in Table 3. According to the results of Table 3, the maximum adsorption rate for bentonite is 238.1 mg/g, and for the optimum adsorbent of ion-exchanged bentonite with magnesium, it is 285.7 mg/g. The synthesized adsorbent has increased the adsorption efficiency by almost 25%, which indicates the high capability of the synthesized adsorbent to absorb the investigated dye.
Effect of contact time
Fig. 10 shows the Removal efficiency of the optimum adsorbent. In the adsorption process, the contact time between the adsorbent and the adsorbed material is one of the effective parameters. The color Removal efficiency and adsorption capacity of the adsorbent are directly related to the contact time of the adsorbent and adsorbate. The adsorption process by the optimum sample is generally divided into two stages: fast adsorption and slow adsorption. According to Figure 10, it can be seen that initially, the adsorption sharply increased and had an upward trend. But, in the sequence, the upward trend continues with a slight slope. So, after 60 mins., a stable equilibrium level has been reached. The reason for this phenomenon is that at the beginning of the adsorption process, the absorbent surface has a large number of empty adsorption sites. As the process continues, the adsorption sites become occupied. By occupying all the active sites, the adsorption should be reduced; as a result, dye is absorbed at a slower rate.
For a better comparison of the results of this research with similar research, Table 4 is presented. As can be seen, most of the adsorbents have quasi-second-order kinetics and obey the Langmuir isotherm, which is in agreement with the present research. The important parameter presented in the table is qm. As can be seen, the maximum absorption by the adsorbent prepared in the present research is much larger than similar adsorbents. Therefore, ion exchange bentonite in the solid phase can be considered as an effective adsorbent for color removal.
CONFLICT OF INTEREST
The authors hereby declare that there is no conflict of interest.
Conclusions
In this work, magnesium exchanged bentonite was prepared and considered to adsorb dye from an aqueous solution. The parent bentonite showed the lowest removal efficiency (40.3%) and prepared magnesium exchanged bentonite for 10 min showed the highest removal efficiency (54.8%). Thus, dye adsorption by modified bentonite increased compared to parent bentonite. After its modification by the ion exchange method, the specific surface was changed from (44.5 to 56.05) m2/g. EDX results indicate that the cation exchange process has been completed successfully. Ion exchange causes some minor changes in bentonite morphology and crystallinity. According to the obtained results, dye adsorption by modified bentonite increased significantly compared to bentonite. the best conditions for removing methylene blue using magnesium exchanged bentonite was pH 10, adsorbent dosage 0.05 mg/l, initial concentration of solution 100 mg/l, and contact time of 120 mins. with a Removal efficiency of 96.67. The Langmuir isotherm had a better correlation coefficient than the Freundlich adsorption model, which indicated the homogeneous surface of the adsorbent.
https://doi.org/10.1007/s10311-018-0786-8
https://doi.org/10.5772/intechopen.90331
https://doi.org/10.1002/9781119526599.ch10
https://doi.org/10.1016/j.matpr.2021.09.119
https://doi.org/10.1080/10643380903218376
https://doi.org/10.1016/j.molstruc.2020.129195
https://doi.org/10.1039/C8RA04290J
https://doi.org/10.1016/j.jiec.2022.09.039
https://doi.org/10.1016/j.jiec.2022.09.039
https://doi.org/10.1016/j.jece.2021.106514
https://doi.org/10.1002/clen.202000189
https://doi.org/10.1016/j.surfcoat.2016.12.032
https://doi.org/10.1016/j.bcab.2021.101965
https://doi.org/10.1021/je100274e
https://doi.org/10.1016/j.matpr.2020.02.403
https://doi.org/10.1016/j.apt.2015.12.006
https://doi.org/10.1016/j.jhazmat.2009.02.097
https://doi.org/10.1007/s41204-016-0010-7
https://doi.org/10.1016/j.matchemphys.2011.05.064
https://doi.org/10.1016/j.clay.2011.04.016
https://doi.org/10.1016/j.cej.2012.01.089
https://doi.org/10.5004/dwt.2021.27311
https://doi.org/10.1016/j.clay.2022.106686
https://doi.org/10.1016/j.molliq.2017.06.115
https://doi.org/10.1016/j.jmrt.2020.05.004
https://doi.org/10.1063/1.4973084
https://doi.org/10.5004/dwt.2022.28367
https://doi.org/10.5004/dwt.2019.24272
https://doi.org/10.1002/ep.14032
https://doi.org/10.1002/sia.1346
https://doi.org/10.1007/s11356-022-19326-4
https://doi.org/10.1016/j.molliq.2021.116373
https://doi.org/10.1016/j.clay.2017.01.021
https://doi.org/10.1155/2017/3039817