Document Type : Original Research Paper
Authors
1 Department of Chemistry, Gauhati University, Guwahati 781014, Assam, India
2 Department of Applied Science and Humanities, Assam University, Silchar 788011, Assam, India
Abstract
Here we report a simple and eco-friendly solvothermal synthesis of graphitic carbon nitride nanospheres (g-CNNS) at 180 oC. The synthesized g-CNNS is characterized by various analytical techniques such as FESEM, PXRD, BET isotherm, Zeta potential, EDX and FT-IR spectroscopy. The adsorption property of g-CNNS is studied using four different dyes in aqueous medium and found that g-CNNS is an efficient material for cationic dye adsorption. A Comprehensive investigation of the kinetics, isotherms and thermodynamics of methylene blue (MB) adsorption, is carried out. The adsorption of MB on g-CNNS is well described by Langmuir isotherm model, and the experimental data fits well with pseudo-second order kinetics. The high rate of adsorption (94.92% MB removal in 120 minute at neutral pH) is attributed to electrostatic interaction between negative charged g-CNNS and cationic organic dye molecule. Additionally, g-CNNS demonstrated good reusability, retaining its efficiency for at least three cycles. Over all our findings suggests that g-CNNS has potential as an efficient adsorbent for wastewater treatment.
Graphical Abstract
Keywords
INTRODUCTION
The release of industrial contaminants into water is contributing to a rise in environmental pollution worldwide, largely due to the rapid expansion of chemical industries [1]. Synthetic dyes such as Methyl orange (MO), Crystal violet, Methylene blue (MB), etc. are discharged from textiles, leather, cosmetics, pharmaceuticals, paint, pulp, and paper industrial processes. Uncontrolled disposal of these contaminated organic dye molecules into water bodies has led to various mutagenic, carcinogenic, and skin diseases in living beings. In addition, these dyes are also creating serious threats to aquatic flora and fauna [2]–[4]. Therefore it is essential to devise a flexible approach to efficiently eliminate dyes from aqueous solutions. Various methods have been applied for the removal of dye compounds from water including ultra-filtration, membrane separation, photocatalytic degradation, electrocatalysis, and adsorption processes [5]–[8]. Among all these methods, adsorption is considered to be the most effective method for removing dye molecules from water systems due to its simplicity in operation, high efficiency, reusability, and cost-effective nature [9], [10]. Several adsorbent materials such as porous hetero-structures, modified activated carbon, exfoliated graphite, and covalent metal-organic framework nanostructures have been explored for the removal of MB from aqueous mediums [9], [11]–[13]. However, some of these materials suffer from slow absorption rates. Therefore, it is necessary to prepare low-cost and efficient adsorbent materials for MB wastewater treatment.
Graphitic carbon nitride (g-C3N4) is a metal-free polymeric material with excellent properties. It exhibits high chemical and thermal stability, making it a durable and environment-friendly material. g-C3N4 and its hybrid structures have recently been applied in photocatalysis, adsorption, energy conversion, and storage [14]–[16]. It is a material with a layered structure similar to graphite but consisting of carbon and nitrogen. Commonly it is synthesized by thermal treatment of nitrogen-rich precursors such as melamine, cyanamide, urea, thiourea, and guanidine hydrochloride etc [17]. g-C3N4 consists of repeating triazene/heptazine units with negatively charged surface due to the presence oxygen oxygen-rich terminal functional groups and nitrogen lone pairs. This makes the g-C3N3 suitable for adsorbing different cationic synthetic dyes and metal ions [18]. George et al. synthesized copper carbon nanofibers-g-C3N4 composite by thermal treatment which is efficient for the removal of MB dye. Its high adsorption capacity is assigned as a negatively charged surface and high surface area [19]. Fronczak et al. synthesized sodium doped g-C3N4 by two-step thermal polycondensation which is found to have extraordinary adsorption capacity for MB via an electrostatic process [20]. Zhang et al. prepared g-CN hydrogel for selective adsorption of organic dyes by the combined effect of electrostatic and π-π interaction [21]. Lu et al. prepared SrCO3/g-C3N4 nanocomposite by a step calcination process and applied it for the adsorption of CV dye from aqueous medium [22]. They suggest the mechanism of adsorption as π-π interaction between g-C3N4 and CV. Thus, it is evident that g-C3N4 is a suitable material to act as an adsorbent for organic dye molecules from water.
In this work, we have successfully prepared graphitic carbon nitride nanospheres (g-CNNS) by a one-step solvothermal method. The morphology, crystallinity, surface area, and surface charge of the g-CNNS are investigated. The layered structure of g-CNNS consists of highly ordered triazene/heptazine units, which facilitates stacking through hydrophobic effects and π-π interactions. The conjugated π region within the g-CNNS layers enables the adsorption of aromatic dye molecules. We have estimated the adsorption property of the g-CNNS using MB, rhodamine B (RhB), MO, and eosin yellow (EY) dye in an aqueous medium under room temperature stirring. The g-CNNS display selective adsorption of cationic MB and RhB dyes over the anionic MO and EY dyes under similar conditions. This is attributed to the presence of “nitrogen pots” within the ordered triazine/heptazine units of g-CNNS, and negatively charged terminal oxygen functional groups. These functional groups facilitate interaction with cationic dye molecules. The zeta potential study has indicated the negative surface charge of the g-CNNS. This endorses the superior adsorption capacity for cationic MB dye at neutral and basic pH compared to acidic conditions. The g-CNNS is found to be very efficient in removing MB dye molecules from aqueous medium, which is a widely used cationic dye known to be harmful to aquatic systems and human health. The novelty of our study lies in the extensive exploration of g-CNNS’ adsorption property, specifically for cationic methylene blue dye which is mentioned below.
- This study focuses relatively less explored adsorption properties of graphitic carbon nitride compared to its well-established role as a photocatalyst. Specifically, the investigation involves the use of graphitic carbon nitride as an adsorbent for cationic dyes, with a particular emphasis on its interaction with methylene blue.
- Highlights of the study include a high adsorption rate and efficiency of 94.92% removal of methylene blue in just 120 minutes at neutral pH. This can be attributed to the dominant electrostatic interaction between the negatively charged g-CNNS and the cationic methylene blue dye molecules.
- A comprehensive exploration of optimal adsorption conditions, including pH, initial dye concentration, temperature, and adsorbent amount.
- In-depth analysis of kinetics, thermodynamics, adsorption capacity, and interaction mechanisms, providing a complete understanding of methylene blue adsorption on graphitic carbon nitride.
EXPERIMENTAL
Materials:
Cyanuric chloride, cyanuric acid, acetonitrile, MB, RhB, MO, and EY are procured from Merck Pvt. Ltd. Distilled water and Whatman-40 filter paper are used for all the experiments.
Methods:
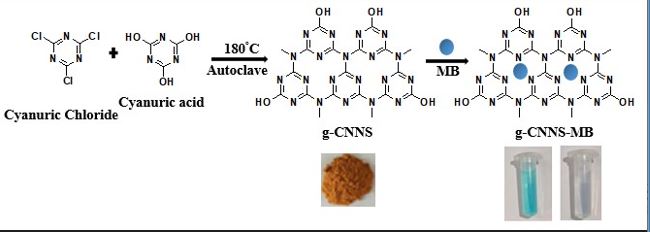
Synthesis of g-CNNS: The graphitic carbon nitride nanospheres are synthesized by following a reported method with modifications [23]. Cyanuric chloride (10 mmol) and cyanuric acid (4 mmol) are dispersed in 30 mL acetonitrile. The obtained mixture is then transferred to an autoclave and heated to 180 oC for 15 hours. On cooling to room temperature, a dark brown product is obtained, which is filtered, washed several times with distilled water, and dried overnight for further use.
Adsorption experiment:
The adsorption of the prepared g-CNNS is studied for four organic dyes. Among them, MB and RhB are cationic dyes while MO and EY are anionic. The chemical structures of the dyes are shown in Fig. 1.
MB shows the highest adsorption efficiency among the four dyes and is selected for further studies. The absorption study is conducted in acidic, neutral, and basic media. To examine the impact of temperature and study the thermodynamic behavior, adsorption at a neutral medium is performed at three different temperatures. In detail, 30 mg of g-CNNS powder is added to a 30 mL dye (10-5 M) solution in a beaker and stirred for 120 minutes. The sample solutions are then collected from the beaker at definite time intervals until adsorption equilibrium is reached. By centrifugation at 1000 rpm for 5 min, the adsorbent is removed from the mixture. The dye concentration is analyzed by monitoring its changing absorption of the band maxima at 664 nm (for MB), 554 nm (for RhB), 464 nm (for MO), and 517 nm (for EY) using a UV-visible spectrometer. Dye removal efficiency is determined using equation (1), which calculates the difference in dye concentration in the aqueous solution before and after adsorption.
(%) Dye removal (1)
Where C0 is the initial and Ce is the equilibrium concentration of the dye solution (mg L-1).
The reusability of the adsorbent is investigated for three cycles. After reaching maximum adsorption, the adsorbent is allowed to settle in the reaction beaker overnight. The next day, the supernatant is removed by decanting and the adsorbent is recovered. It is then washed several times with water and dried. Before each subsequent cycle of adsorption, the dried adsorbent is collected and weighed.
Instrumentation:
The sample morphology size and elemental composition are examined using a ZEISS Sigma-300 field emission scanning microscope (FESEM) equipped with an energy-dispersive X-ray (EDX) spectrometer. A Rigaku Ultima IV X-ray diffractometer with CuKaα radiation (λ=1.54 Å) is used for recording the powder X-ray diffraction patterns from 5o to 80o (2θ). SHIMADZU, UV-1800, and IR Affinity-1 spectrometers are used for recording UV-visible and FTIR spectra respectively. The surface charge and surface area of the samples are measured by NICOMP ZLS zeta potential analyzer. Quantachrome Instruments’ NOVA 1000E respectively.
RESULTS AND DISCUSSION
Scheme 1 illustrates the synthesis of g-CNNS through the condensation polymerization of cyanuric chloride and cyanuric acid in an oven at 180 oC, as well as its application as an adsorbent for the removal of MB dye. The morphology and size of g-CNNS are analyzed by FESEM. As evident from the FESEM image in Fig. 2a, the g-CNNS are spherical, and particle size distribution ranges from 180 nm to 450 nm [23]. PXRD pattern of g-CNNS shows a relatively intense peak at 27.9o (200) 2θ due to the layered graphitic structure with an interlayer spacing of 0.320 nm, similar to earlier reports, [24], [25] Fig. 2b. We have analyzed the chemical and elemental composition of the g-CNNS using FT-IR, Fig. 2c and EDX Fig. 2d spectroscopy respectively. Broad peak observed at 3444 cm-1, Fig. 2c is assigned to the stretching vibration of-NH2. Peaks observed at 1425 cm-1 and 1559 cm-1 are assigned to C-N and C=N stretching vibrations respectively [14]. The characteristic peak observed at 804.7 cm-1 is attributed to the triazine ring of the g-CNNS. The peak observed at 2364 cm-1 is due to C-C=N vibrational mode [24], [25]. As evident from the EDX spectrum, Fig. 2d (inset: corresponding elemental composition table), the elemental weight percentage of g-CNNS is determined as C = 24.95 %, N = 56.27 %, and O = 18.78 % confirming the formation of graphitic carbon nitride along with some oxygen-containing terminal functional groups.
Organic dyes such as MB, RhB, EY, and MO are toxic pollutants found in water sources due to industrial disposal. Among these organic dyes, it is observed that g-CNNS is a highly efficient adsorbent for the removal of MB. Fig. 3a shows the UV-visible absorption spectrum of 10-5 M MB (initial concentration) in aqueous medium. The absorbance maxima monitored at 664 nm decrease with time from 0 to 120 min, indicating the effective removal of MB from the solution. Complete discoloration of MB solution, Fig. 3b at 120 min also shows efficient MB removal. A comparison of % removal of different dyes by g-CNNS is shown in Fig. 3c. The g-CNNS removes 94.08 % of MB within 90 min (with minimal increase up to 94.92 % observed till 120 min) Fig. 3c, black line. For another cationic dye RhB, the removal percentage by g-CNNS is 35.4 % within 120 min (with up to 42.81% removal observed till 240 min) Fig. 3c, red line. While for anionic dyes EY and MO, the % removal is less, 4.35 % and 2.45 % respectively in the same period of 120 min, Fig. 3c, green and blue lines. The UV-visible absorption spectra corresponding to the removal of RhB, EY, and MO are included in the supporting information, Fig. S1 (a)-(c). Initial concentrations for all dyes are maintained at 10-5 M with 30 mg of gCNNS in 30 mL of water. Therefore, it is clear that g-CNNS have higher adsorption efficiency for cationic dyes compared to anionic dyes, which is due to the negatively charged surface of g-CNNS. The negative surface charge of -45.59 mV, Fig. 3d supports the assessment that the adsorption of MB is due to ionic interactions between the negatively charged or electron-rich nitrogen species on g-CNNS and the positively charged functional groups on the dye molecules. However, the lower adsorption of RhB may be attributed to its bulkier structure compared to MB, Fig. 1. Smaller-sized MB can more easily diffuse into the layered structure of g-CNNS. A study conducted by S Wang et al. on the adsorption of three cationic dyes, namely MB, CV, and RhB, using MCM-22 as the adsorbent, revealed similar results [26]. Their results showed that the adsorption followed the order of MB > CV > RhB, indicating that MB had the highest affinity towards MCM-22, followed by CV and RhB. S. Eftekhari et al. studied MB and RhB adsorption on AlMCM-41 and reported similar results [27].
As the adsorption of MB on g-CNNS has shown superior performance compared to other dyes, we have conducted further investigations using MB as a model dye to explore the factors that can influence this adsorption process. These investigations include studying the effect of the adsorbent amount, pH of the MB solution, reaction temperature (which is used to calculate thermodynamic parameters), initial MB concentrations, and conducting studies on the kinetics and isotherms of the adsorption process. For consistency, the concentration of MB is expressed in parts per million (ppm) in these experiments. The initial concentration of 10-5 M that has been used so far is equivalent to 3 ppm for MB when converted to ppm.
The removal percentage of MB increases with increasing amount of g-CNNS from 10 mg to 30 mg, Fig. 3e that is due to the increment of active sites. However, a further increase in g-CNNS amount to 40 mg does not show any improvement in MB removal percentage, indicating that 30 mg in 30 mL is the optimal adsorbent amount to achieve maximum adsorption. The MB removal % with different amounts of g-CNNS are 10 mg = 28.50 %, 20 mg = 47.20 %, 30 mg = 94.92 % and 40 mg = 91.29 %. To compare the results, all the data are considered at 120 min. However, in the case of 10 mg and 20 mg, adsorption is checked up to 240 and 180 min, and respective adsorption spectra are given in the supporting information Fig. S2. The highest percentage of MB removal (94.92 %) is observed with 30 mg of g-CNNS within 120 min, which is comparable or superior to previous reports [28]–[32]. A comparison table is included in the supporting information (Table S1 in supporting information). This table provides a detailed overview of the adsorption capacities of various graphitic carbon nitride samples prepared under different conditions, reported in the literature. The specific surface area of the g-CNNS is determined by N2 adsorption-desorption isotherm, Fig. 3f. The BET surface area of the g-CNNS is found to be 5.71(m²/g) which provides a good surface for adsorption of MB molecules.
To investigate the influence of pH on g-CNNS and MB, the adsorption experiment is carried out under three different pH conditions, acidic (pH = 1.6), ~neutral (pH = 6.7), and basic (pH = 10.6). As evident from Fig. 4a, the MB removal capability is found to be highest at pH 6.7 (94.92%) and remained nearly the same at pH 10.6 (94.35%), while decreasing at pH 1.6 (33.68%). Corresponding UV-visible spectra of MB adsorption at acidic (pH = 1.6) and basic (pH = 10.6) conditions are given in the supporting information Fig. S3. Similar MB removal capability in both neutral and basic conditions is attributed to the free negatively charged surface of g-CNNS, facilitating the adsorption. On the other hand at pH 1.6, the MB removal efficiency is significantly decreased by protonation of the g-CNNS surface, thus blocking the binding sites in MB. Furthermore, we conducted adsorption experiments at three different temperatures specifically 303, 323, and 343 K. The impact of temperature on MB removal is illustrated in Fig. 4b, showing a slight decrease in the percentage removal of MB with increasing temperature. The MB removal percentages at three different temperatures are 303 K = 94.92%, 323 K = 91.86%, and 343 K = 91.29%. The UV-visible spectra of MB adsorption on g-CNNS at 323 and 343 K are given in Fig. S4 in the supporting information. This little decrease in the percentage removal of MB on g-CNNS with increasing temperature might be due to increased agitation in the medium. This random movement of MB molecules makes them less available for fixed active sites on g-CNNS resulting in a little decrease in adsorption. The decrease in percentage removal with increasing temperature indicates the exothermic nature of the adsorption process, which is supported by the values obtained for the thermodynamic parameters. The relation between the free energy change ∆G0, change in enthalpy ∆H0, and change in entropy ∆S0 is as follows:
The adsorption equilibrium constant Kc can be calculated by taking the ratio of the amount of MB adsorbed at equilibrium (qe) to the concentration of dye solution at equilibrium (Ce).
The equation used to calculate the equilibrium uptake is as follows:
The initial concentration of the dye solution is denoted by C0 (mg L-1), while Ce (mg L-1) represents the equilibrium concentration of the dye solution. V (L) refers to the volume of the dye solution and W (g) denotes the mass of adsorbent utilized.
Gibbs free energy can be calculated using the following equation, which utilizes the value of Kc:
The van’t Hoff equation can be derived based on the above equations:
Values of ∆H0 and ∆S0 are obtained from the slope and intercept of the plot of ln Kc versus 1/T, respectively. The van’t Hoff plot for the adsorption of MB on g-CNNS is presented in Fig. S5 (supporting information) and the calculated thermodynamic parameters are summarized in Table 1. The negative values of ∆H0 (-12.68 KJmol-1) and negative values of ∆G0 confirm that the adsorption process is exothermic and spontaneous. The negative value of ΔS0 (-18.02 JK−1mol−1) suggests a decrease in system entropy, indicating a transition towards a more ordered state through the adsorption process. This increase in order can be attributed to the establishment of ionic interactions between the positively charged methylene blue molecules and the negatively charged surface of g-CNNS. A negative value of ∆S0 also indicates that the adsorption process does not lead to a significant change in the internal structure of the adsorbent [33].
The influence of the initial dye concentration on the adsorption process is studied for MB from 3 ppm to 9 ppm while keeping the amount of g-CNNS fixed at 30 mg. The results of these experiments, depicted in Fig. 4c (left axis), show the percentage of MB removal. Corresponding UV-visible spectra can be found in Fig. S6 of the supporting information. The maximum dye adsorption capacity of the g-CNNS is also calculated at the same fixed amount, Fig. 4c (Right scale). As observed from Table 2, on varying the MB concentration from 3 ppm to 9 ppm, the % removal of MB decreases from 94.92 % to 77.75 %, which is due to the increase in the number of dye molecules with a fixed amount of adsorption sites, however, the adsorption capacity of g-CNNS increases from 2.75 (mgg-1) to 7.41 (mgg-1), due to the increase in the number of dye molecules [28]. With the increase of MB concentration, the number of dye molecules increases in a fixed volume of dye solution which generates an internal pressure among the dye molecules, and some of the dye molecules are forced to diffuse to the inner layers of g-CNNS. Initially, g-CNNS adsorbs MB molecules on its surface, and with the increase of MB concentration, adsorption also occurs in the inner layers of g-CNNS, leading to an increase in its adsorption capacity, relevant experimental details are discussed later.
To investigate the adsorption kinetics followed, the applicability of pseudo-first and pseudo-second-order models are examined using linear expression [32], [34]. The pseudo-first-order model, represented by equation (7), describes the adsorption of a solute from a solution.
In the above equation, qt (mgg-1) and qe(mgg-1) represent the amount of MB adsorbed at time t and at equilibrium respectively, while k1 is the rate constant of pseudo first order adsorption (min-1).
The second-order model for adsorption is based on the adsorption capacity of the solid phase and is expressed by equation (8).
In the above equation, ks (mgg−1min−1) represents the rate constant, while qt (mgg-1) represents the amount adsorbed at time t.
The plots of the pseudo-first and pseudo-second-order kinetic models at different MB dye concentrations are shown in Fig. 5a and 5b. The rate constants of both models, along with their correlation coefficients, are presented in Table 3. Although both models fit the data well (with correlation coefficients close to 1), the pseudo-second-order model is more suitable for describing the adsorption process, as the experimental data fits better to this model. The adsorption of MB on g-CNNS follows 2nd-order kinetics suggesting that the process of adsorption is dependent on both the initial concentration of dye solution and the amount of adsorbent. [35].
The pseudo-second-order kinetics model takes into account the diffusion process by incorporating a term that accounts for the square of the difference between the equilibrium adsorption capacity and the adsorption at a specific time. This term reflects the gradual slowdown of adsorption as the system approaches equilibrium, indicating the diffusion-controlled nature of the process. To evaluate the individual steps involved in the adsorption of MB on g-CNNS, we have considered the intra-diffusion model, which is expressed by Equation (9) [36]. By utilizing this model, we can gain a better understanding of the diffusion dynamics and the rate-controlling factors during the adsorption process.
Where ki is the intra-particle rate constant and C is the intercept. From the Figs. 5c and 5d, it is clear that the adsorption process occurs in two steps. The initial linear portion of the plots represents the electrostatic attraction between MB and g-CNNS, while the final portion represents the intraparticle diffusion and the gradual slowdown of adsorption reaching equilibrium. The first step involves the diffusion of MB molecules through the bulk of the solution to reach the surface of the adsorbent, where they adsorb at the active sites. In the second step, after the adsorption on the surface of g-CNNS, a few MB molecules diffuse to the inner layers of g-CNNS and adsorb at the active sites within these layers. These steps are illustrated in Scheme 2. Our experimental findings support this, as we observed removal percentages of 74.36% and 61.65% within 15 and 45 minutes, respectively, for initial MB concentrations of 3 and 9 ppm. These removal percentages account for 78% and 79% of their respective total removal percentage and are achieved rapidly. The initial step of adsorption is typically very fast due to the strong electrostatic interaction between the cationic MB and the negatively charged surface of g-CNNS, facilitating rapid binding. However, the adsorption process then gradually becomes slower and more stable, eventually reaching equilibrium with maximum adsorption [37].
The adsorption of MB dye to the g-CNNS are fitted to two different adsorption models Langmuir and Freundlich isotherms shown in equation (10) and (11) [34], [38]. Linear regression is used to determine the best-fitting model.
Where KL (Lmg-1) is the Langmuir adsorption constant, KF and n are Freundlich constants, and qmax (mgg-1) is the maximum adsorption capacity of the adsorbent. The constants are determined by analyzing the intercept and slope of a linear plot of the experimental data, as shown in Figs. 6a and 6b. The obtained data are shown in Table 4. Based on these data, it is evident that the Langmuir isotherm (R2 = 0.9899) provides the best fit for the MB adsorption on g-CNNS, with an adsorption capacity of 8.62 mgg-1. The Langmuir isotherm model suggests that the adsorbent surface has a uniform distribution of active sites and that the adsorption process involves a monolayer coverage of MB dye on the surface of g-CNNS.
Adsorption mechanism:
The adsorption mechanism of methylene blue onto g-CNNS involves a combination of electrostatic interactions, hydrogen bonding, and π-π stacking interactions. These interactions collectively contribute to the binding and adsorption of MB onto the g-CNNS surface. Graphitic carbon nitride possesses primary, secondary, and tertiary amine groups on its surface, which can undergo ionization in an aqueous suspension. This ionization is expressed by the following reactions:
At low pH conditions, the amine groups acquire positive surface charges due to an excess of protons, as expressed by reactions (1-3). This positive charge results in the repulsion of positively charged MB molecules, leading to a decrease in adsorption under acidic pH conditions. Conversely, at basic pH conditions, the primary and secondary amine groups react with hydroxyl ions, causing the g-CNNS particles to become negatively charged, as expressed by reactions (4) and (5). The surface charge behavior of g-CNNS plays a significant role in the adsorption process.
To further investigate the surface charge behavior, additional zeta potential measurements are conducted under varying pH conditions, as depicted in Fig. 7 (a-c). These measurements, performed at pH 1.6 (43.49 mV), pH 3 (28.78 mV), and pH 6.7 (-45.59 mV), clearly demonstrate a distinct shift in surface charge as the pH changes, indicating significant alterations within the investigated pH range. The obtained data strongly suggests that the point of zero charge (PZC) for the g-CNNS likely exists within the pH range of 3 to 6.7, with a probable PZC value estimated at pH 4.4, as depicted in Fig. 7d. This shift towards a negative surface charge promotes electrostatic interactions between the negatively charged g-CNNS surface and the positively charged MB, thereby enhancing adsorption.
Apart from electrostatic interactions, the hydroxyl groups on the g-CNNS surface can engage in hydrogen bonding with the MB molecules, creating attractive forces between the hydroxyl groups of graphitic carbon nitride and the amino groups or other hydrogen bond acceptors present in the MB structure. These hydrogen bonds facilitate the interaction and adsorption of MB onto g-CNNS. Furthermore, the conjugated π-electron system of graphitic carbon nitride enables it to undergo π-π stacking interactions with the aromatic rings of MB. The delocalized π-electrons of g-CNNS interact with the π-electrons of the aromatic rings in MB, providing additional attractive forces for adsorption and enhancing the binding between g-CNNS and MB.
The successful adsorption of MB on g-CNNS is supported by the FTIR spectra recorded after adsorption, as shown in Fig. S7 in the supporting information. The observed broadening of peaks in the range 3410-3038 cm-1 and 1690-1310 cm-1 in the FTIR spectra after MB adsorption on g-CNNS provides clear evidence of effective adsorption of MB molecules onto the g-CNNS surface [39]. Specifically, the broadening of the peak in the range 3410-3038 cm-1 suggests the interaction of MB functional groups with -NH2, and -NH groups on g-CNNS upon adsorption, possibly through ionic interactions or hydrogen bonding. The FTIR spectra of pure MB exhibit characteristic peaks in the range of 1603 to 1394 cm-1, which correspond to vibrational modes associated with the aromatic ring structures present in methylene blue [40]. On the other hand, in g-CNNS, the peaks observed at 1425 cm-1 and 1559 cm-1 are assigned to the C-N and C=N stretching vibrations, respectively. When MB is adsorbed onto g-CNNS, the broadening of peaks in the range 1690-1310 cm-1 suggests changes in the bonding of both MB and g-CNNS due to interaction between them. This broadening can be attributed to the interaction between the aromatic ring structures of MB and g-CNNS, potentially involving π-π interactions or other forms of molecular interactions [39].
The reusability of the adsorbent is an important factor to consider in real-time applications. Hence, the g-CNNS is reused for further adsorption of MB from an aqueous medium for three repeated cycles. The efficient reuse of g-CNNS as an adsorbent is indicated by the almost identical MB removal per milligram of the adsorbent in the three subsequent cycles, as shown in Fig. 8 and Table 5. In each repeat cycle, the MB removal percentage decreases due to the loss of adsorbent in the recovery process but the adsorption efficiency remains the same.
CONCLUSION
In summary, g-CNNS has been synthesized by a simple solvothermal method using cyanuric chloride and cyanuric acid as the precursors. The obtained g-CNNS has good and selective adsorption capability of cationic dye MB than the anionic dye from aqueous medium within 120 min. The strong electrostatic interaction between the negatively charged surface of g-CNNS and the positively charged cationic MB is responsible for the effective adsorption of MB on the surface of the adsorbent. The adsorption ability of the g-CNNS is very effective both in a neutral and basic medium in comparison to an acidic medium. Additionally, the percentage of MB removal increases as the amount of g-CNNS is increased from 10 mg to 30 mg, but after that, it remains stable, indicating that 30 mg of g-CNNS in 30 mL of solution is the optimal adsorbent amount for achieving maximum adsorption. The assessment of thermodynamic parameters indicates that the adsorption process is both exothermic and spontaneous. The experimental data is well-fitted with the pseudo-second-order kinetics model. The adsorption process is best described by the Langmuir model and the Langmuir adsorption capacity is determined to be 8.62 mgg-1. The g-CNNS can adsorbed about 94.92 % of the MB within 120 min and can be repeated for at least three cycles. Hence, these g-CNNS can be applied as eco-friendly and promising adsorbent materials for the efficient removal of MB from wastewater.
Acknowledgement:
PS and S.K.G are thankful to the SERB-DST SB/S1/PC-105/ 2012, Govt. of India, for financial assistance. The authors also would like to thank CIF, Gauhati University, and DST-FIST support to the Department of Chemistry, Gauhati University for instrumental support.
CONFLICT OF INTEREST
The authors hereby declare that there is no conflict of interest.