Document Type : Original Research Paper
Authors
1 Chemistry Department, Faculty of Science, Beirut Arab University, Beirut, Lebanon
2 Physics Department, Faculty of Science, Beirut Arab University, Beirut, Lebanon
3 Physics Department, Faculty of Science, Alexandria University, Alexandria, Egypt
Abstract
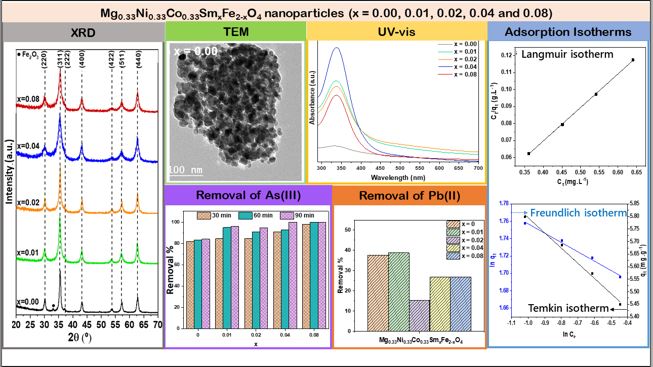
Heavy metal ions, such as As (III) and Pb (II), are harmful even at trace levels and have caused series health effects on living beings. Therefore, removing these heavy metal ions from the aqueous environment is highly desirable. In this study, Mg0.33Ni0.33Co0.33SmxFe2-xO4 nanoparticles, where x = 0.00, 0.01, 0.02, 0.04 and 0.08, were synthesized by the co-precipitation method and characterized using X-Ray Diffraction (XRD), Transmission Electron Microscopy (TEM) and UV-Vis spectroscopy techniques in order to study the structural and optical properties. The prepared nanoparticles were applied as adsorbents for the removal of As (III) and Pb (II) from wastewater. Among the prepared samples, Mg0.33Ni0.33Co0.33SmxFe2-xO4 nanoparticles with x = 0.04 and 0.08 exhibited improved adsorption performance where As (III) was totally removed after 90 min. The experimental adsorption data of As (III) was well fitted with a second-order kinetics model and Langmuir isotherm. Furthermore, the highest removal % of Pb (II) was revealed by Mg0.33Ni0.33Co0.33SmxFe2-xO4 nanoparticles with x = 0.01. Thus, doping Mg0.33Ni0.33Co0.33Fe2O4 with Sm improved the adsorption performance of nanoparticles for the removal of As (III) more than that of Pb (II).
Graphical Abstract
Keywords
INTRODUCTION
With the expansion of industry and human activities, such as plating, batteries, pesticides, electroplating, and mining, excessive amounts of heavy metal ions and organic pollutants are found in wastewater [1-3]. Lead (Pb), arsenic (As), mercury (Hg), cadmium (Cd), and chromium (Cr) are the most often used heavy metals [4]. Being non-biodegradable and carcinogenic, the presence of heavy metal ions in water in inappropriate concentration poses a serious risk to the health of all living things [5]. Since heavy metal ions endanger both human health and the ecosystem their removal from water is highly desirable. Consequently, several technologies have been used to remove heavy metal ions, including adsorption, chemical precipitation, ion exchange, and membrane filtration [6-8]. Among the previously listed techniques, adsorption is one of the most often applied techniques due to its low cost and ease of usage [9, 10].
The majority of adsorbents, used for the removal of heavy metal ions, are difficult to separate after the treatment process since complicated procedures like filtration or centrifugation are required [11]. However, the usage of magnetic adsorbents solves the separation problem since the adsorbents can be simply separated by applying an external magnetic field. Spinel ferrite nanoparticles have been expansively used in various environmental applications such as the degradation of toxic pollutants and the removal of heavy metal ions [12-15]. This is owed to their small particle size, dispersity, large surface area, and magnetic properties that permit their magnetic separation [16]. Khoso et al. reported the following removal efficiencies of 89 % of Cr(VI), 79 % of Pb (II), and 87 % of Cd(II) by NiFe2O4 nanoparticles [17]. Moreover, Ca-doped Ni0.4Zn0.6Fe2O4 nano-ferrites removed 98.25 % of Cd and 57% of Cr ions [18].
Doping spinel ferrite nanoparticles with rare earth metals improves their properties [19, 20]. Based on the above background, this work evaluates the adsorption performance of Mg0.33Ni0.33Co0.33SmxFe2-xO4 nanoparticles where x = 0.00, 0.01, 0.02, 0.04, and 0.08 for the removal of As (III) and Pb (II).
METHODS AND MATERIALS
Synthesis of Nanoparticles
Mg0.33Ni0.33Co0.33SmxFe2-xO4 nanoparticles, where x = 0.00, 0.01, 0.02, 0.04, and 0.08, were synthesized by the co-precipitation technique. Appropriate amounts of CoCl2.H2O, NiCl2.6H2O, MgCl2.6H2O, SmCl3.6H2O, and FeCl3.6H2O were dissolved in a specific volume of deionized water. To prepare 25 g of Mg0.33Ni0.33Co0.33Fe2O4 nanoparticles, 2.58, 2.59, 2.21, and 17.6 g of CoCl2.H2O, NiCl2.6H2O, MgCl2.6H2O, and FeCl3.6H2O, respectively, were dissolved in deionized water. For the synthesis of Mg0.33Ni0.33Co0.33SmxFe2O4 nanoparticles where 0.01 ≤ x ≤ 0.08, the amount of SmCl3.6H2O and FeCl3.6H2O ranges between 0.06 and 0.47 g and between 8.38 and 8.76 g, respectively. Then, the prepared solutions were mixed and stirred magnetically for 30 minutes at room temperature. Afterward, 60 g of sodium hydroxide (NaOH) was dissolved in 0.5 L of deionized water to prepare a 3 M solution of NaOH which was added to the mixture drop by drop till reaching pH 12. Then, while stirring magnetically, the resulting solution was heated at 80 °C for 2 hours. The precipitated products were filtered and washed until the pH reached 7. The precipitates were dried for approximately 18 hours at 100 °C. Finally, dried powders were then annealed at 550 °C for 4 hours.
Characterization of Mg0.33Ni0.33Co0.33SmxFe2-xO4 Nanoparticles
The structural properties of the prepared samples were determined by X-ray diffraction (XRD) and transmission electron microscopy (TEM). Thus, Bruker D8 Focus was employed to do the XRD analysis using Cu-ka radiation source (𝜆 = 1.54056 Å) in the range of 20° ≤ 2q ≤ 80°. The morphology and particle size were evaluated by TEM via JEM-1400 Plus. The optical properties of the prepared samples were evaluated by ultraviolet-visible (UV-Vis) spectroscopic measurements that were conducted in the range of 290 - 700 nm using a V-670 spectrophotometer. The magnetic properties were examined at room temperature by a Lakeshore 7410 vibrating sample magnetometer (VSM) applying a magnetic field in the range of -20000 and +20000 G.
Adsorption Performance of Mg0.33Ni0.33Co0.33SmxFe2-xO4 Nanoparticles
The adsorption performance of Mg0.33Ni0.33Co0.33SmxFe2-xO4 nanoparticles, where 0.00 ≤ x ≤ 0.08, was evaluated for the removal of As (III) and Pb (II). The desired concentrations of the As (III) and Pb (II) solutions, namely 5 mg.L-1 for As (III) and 5, 7.5, and 10 mg.L-1 for Pb (II), were prepared through dilution of the corresponding stock solutions. It is important to note that the stock solutions for As (III) and Pb (II), purchased from Sigma-Aldrich, have a concentration of 1000 mg.L-1 each. The pH of 5 mg.L-1 As (III) and Pb (II) solutions are 2.69 and 2.64, respectively. To determine the most efficient sample in the removal of As (III) and Pb (II), 40 mg of each of the prepared samples was mixed with 50 mL of 5 mg.L-1 As (III) and Pb (III) solutions. The mixtures were sonicated at room temperature. After a predetermined time interval, the concentration of heavy metal ions was determined by atomic absorption spectroscopy (AAS). The adsorption kinetics and isotherm for the removal of As (III) were determined. Furthermore, the effect of the adsorbent amount and initial concentration of Pb (II) on the removal % of Pb (II) was studied. The removal % and adsorption capacity (qe) were calculated using the following formulas:
where C0 (mg.L-1 ) and Ce (mg.L-1) denote the concentration of As (III) at t = 0 and equilibrium respectively, V (L) is the volume of As (III) solution, and m (g) is the adsorbent mass.
RESULTS AND DISCUSSION
Structural, Optical, and Magnetic Properties of Mg0.33Ni0.33Co0.33SmxFe2-xO4 Nanoparticles
The XRD patterns for Mg0.33Ni0.33Co0.33SmxFe2-xO4 nanoparticles where 0.00 ≤ x ≤ 0.08 are displayed in Fig. 1. The observed peaks are indexed to cubic spinel phase with space group Fd3m and are well matched with the standard JCPDS card no. 36-0398 [21]. The presence of an extra peak in the XRD pattern confirms the presence of hematite as a secondary phase. However, the intensity of this peak decreases as the Sm content increases. The decrease in intensity of the hematite peak might be attributed to the occupation of Sm3+ ions in the host lattice. Thus, the absence of extra peaks of dopants or secondary phases revealed the successful replacement of iron ions by the dopant ions (Sm3+). Similar results are reported in a previous study upon doping Co0.9Cu0.1Fe2O4 with Dy3+ and Sm3+ [22]. The lattice parameter (a) values of Mg0.33Ni0.33Co0.33SmxFe2-xO4 nanoparticles where 0.01 ≤ x ≤ 0.08 are greater than that of pure nanoparticles (x = 0.00) as listed in Table 1. This is mainly attributed to the substitution of Fe3+ by Sm3+ ions which is driven by the significant difference in ionic radii knowing that Sm3+ ions have a larger ionic radius (1.08 Å) compared to that of Fe3+ (0.65 Å). Liu et al. [23] reported similar results where the lattice parameter increased from 8.381 to 8.411 Å upon doping Ni0.5Zn0.5Fe2O4 with Sm.
TEM images and particle size distribution of Mg0.33Ni0.33Co0.33SmxFe2-xO4 nanoparticles are displayed in Fig. 2. The prepared samples exhibit spherical morphology. In addition, as Sm content rises from 0.00 to 0.08, the average particle size is reduced from 28.72 to 21.66 nm. It is known that the rare earth metal ions substitution shrinks the particle size of ferrite nanoparticles as reported in previously published studies [24, 25]. The particle size reduction is attributed to the substitution of Fe3+ with Sm3+. The diffusion of Sm3+, having a larger ionic radius than Fe3+, into Mg0.33Ni0.33Co0.33Fe2O4 grains arises upon doping nanoparticles with Sm. Thus, the surface energy is reduced and the growth of the nanoparticles is inhibited owed to the segregation of Sm3+ to the surface. Similar results were reported in a previous study where the particle size was reduced from 60 to 32 nm as the Sm content increased from 0 to 0.1 in CoSmxFe2-xO4 nanoparticles [26].
The specific surface area of nanoparticles plays a vital role in their versatility and applicability across various fields such as catalysis and heavy metal adsorption. To determine the specific surface area (S) of the synthesized nanoparticles, the following relation was employed [27]:
where ρ and D represent the X-ray density and the average particle size obtained from TEM analysis, respectively. It is worth mentioning that the X-ray density (ρ) was calculated using the following equation [27]:
knowing that M denotes the molar mass, NA represents Avogadro’s number, and a signifies the lattice parameter of the prepared nanoparticles. The obtained values of ρ and S are listed in Table 1. Notably, the specific surface area (S) increases upon doping nanoparticles with Sm. Among the prepared samples, Mg0.33Ni0.33Co0.33SmxFe2-xO4 nanoparticles with x = 0.04 exhibited the largest specific surface area (S = 54.356 m2/g)
The optical properties of Mg0.33Ni0.33Co0.33SmxFe2-xO4 nanoparticles, where x ranges between 0.00 and 0.08, are determined via UV-vis spectroscopy. The absorption spectra, depicted in Fig. 3, were recorded in the range of 290 - 700 nm and a major peak appeared at 335 nm. Comparable UV spectra of Ni1-xMgxFe2O4 nanoparticles are stated in previous studies [28].
The bandgap energy (Eg) was estimated from Tauc’s plot by applying the following equation:
where α denotes the absorption coefficient, hυ is the photon energy and A represents the transition probability constant. Thus, the bandgap energy (Eg) was assessed by plotting (αhυ)2 versus hυ, as displayed in Fig. 4 and Table 2. The Sm-doped nanoparticles revealed greater Eg values compared to that of pure Mg0.33Ni0.33Co0.33SmxFe2-xO4 (x=0.00). As revealed from TEM analysis, the Sm-doped samples revealed smaller particle sizes compared with pure nanoparticles. Thus, the decrease in the particle size and increase in the bandgap energy upon Sm-doping is owed to the quantum size effect [29].
The Urbach energy (EU), related to the disorder or defect in the prepared samples, is estimated from the following equation:
knowing that α0 is constant. Thus, EU is determined from the reciprocal slope of the plot of ln (α) vs. (hν). The obtained results are displayed in Fig. 5 and EU values are listed in Table 2. The doped samples exhibited lower EU than pure nanoparticles. Furthermore, an inverse proportionality between EU and Eg was noticed. Similar results were reported in a previous study upon doping ZnFe2O4 and thin films of PVA with La and SrCuTi2Fe14O27, respectively [30, 31].
The extinction coefficient (𝑘) related to the absorption coefficient (𝛼) can be determined by the following equation [30]:
The variation of the extinction coefficient (𝑘) as a function of wavelength (𝜆) for the prepared samples is illustrated in Fig. 6 (a). The extinction coefficient (k) increases to its maximum value around 335 nm and then decreases. Furthermore, the extinction coefficient of Sm-doped samples is greater than that of pure nanoparticles. This might be attributed to the increase in the absorption coefficient revealing the occurrence of direct electronic transitions [30, 32].
The refractive index (n) is an important characteristic in determining the optical and electric properties of a semiconductor [33]. It can be calculated from the following equation:
where TS is the percentage transmission coefficient. The variation of the refractive index (n) against wavelength for Mg0.33Ni0.33Co0.33SmxFe2-xO4 nanoparticles with 0.00≤x≤0.08 is represented in Fig. 6 (b). The refractive index increases as the wavelength increases to reach 335 nm. With a further increase in the wavelength to reach 700 nm, the refractive index decreases. Furthermore, the refractive index is greatly increased upon doping the nanoparticles with Sm. Hence, doping Mg0.33Ni0.33Co0.33SmxFe2-xO4 nanoparticles plays an important role in controlling the k and n values, helps in enhancing the optical properties of nanomaterials and enables its usage in optoelectronic applications [30].
The prepared nanoparticles were subjected to VSM analysis to investigate their magnetic properties. The obtained M-H loops are displayed in Fig. 7. Ferromagnetic behavior was exhibited by Mg0.33Ni0.33Co0.33SmxFe2-xO4 nanoparticles with x = 0.00, 0.01 and 0.08. Furthermore, the values of Ms and Mr are reduced from 31.87 to 15.31 emu/g and from 8.31 to 1.72 emu/g, respectively, as Sm content increases from 0.00 to 0.08. In addition, the Hc values are reduced from 284.89 to 199.70 G upon doping nanoparticles with Sm. The reduction in Ms and Mr can be attributed to the introduction of Sm3+ ions into the nano ferrite lattice, which replaces some of the Fe3+ ions. This substitution leads to a dilution of the magnetic moment, as the magnetic moment of Sm3+ (1.7 μB) is smaller than that of Fe3+ (5 μB) [34]. Consequently, the overall magnetic moment of the doped nanoparticles decreases, resulting in a decrease in the Ms values.
Adsorption Performance of Nanoparticles for the Removal of As (III)
The efficiency of Mg0.33Ni0.33Co0.33SmxFe2-xO4 nanoparticles with 0≤x≤0.08 for the removal of As (III) was evaluated. The removal % of As (III) as a function of contact time in the presence of nanoparticles with various Sm content is shown in Fig. 8. As the contact time increases from 30 to 90 min, the removal % of As (III) increases. Among the prepared samples, Mg0.33Ni0.33Co0.33SmxFe2-xO4 nanoparticles with x = 0.04 and 0.08 exhibited improved performance where 100 % of As (III) was removed after 90 min. Thus, the adsorption activity of Mg0.33Ni0.33Co0.33Fe2O4 nanoparticles is boosted with Sm doping. Enhanced adsorption performance was exhibited by Mg0.33Ni0.33Co0.33SmxFe2-xO4 nanoparticles with x = 0.04 and 0.08 compared with magnetite–maghemite nanoparticles; 98.5 % of As (III) was removed in the presence of 0.4 g.L-1 of magnetite–maghemite nanoparticles after a contact time of 24 hours knowing that the starting initial As (III) concentration was 1.5 mg/L and the solution pH = 2 [35].
Adsorption kinetics
To study the adsorption kinetics, 40 mg of Mg0.33Ni0.33Co0.33SmxFe2-xO4 nanoparticles with 0≤x≤0.08 was mixed with 50 mL of 5 mg.L-1 As (III) solution having pH = 2.69. The adsorption kinetics were analyzed at room temperature. To determine the kinetic model that best fits the adsorption experimental results of As (III), the first-order, second-order, Elovich, and intra-particle diffusion (IPD) models were applied. The first-order model is represented in the following equation:
However, the second-order model is represented as follows [36]:
where qt and qe represent the adsorption capacity at any time t and equilibrium (mg.g-1), k1 denotes the first-order rate constant (min-1) and k2 represents the second-order rate constant (g.mg-1.min-1). The Elovich kinetic model is an empirical equation used to describe the adsorption kinetics and assumes that the rate of adsorption is influenced by both the surface coverage and the activation energy of the adsorption process. The Elovich equation can be written as [37]:
where qt is the amount of solute adsorbed at time t, β is the desorption constant, which represents the desorption energy or activation energy of the adsorption process and α is the initial adsorption rate constant. The intra-particle diffusion (IPD) model, is used to analyze the adsorption process and assumes that the adsorption occurs in multiple stages, including external mass transfer, intra-particle diffusion, and equilibrium. The IPD model can be represented by the equation [38]:
where k represents the IPD rate constant and I is the IPD model’s boundary layer constant.
The equations and the coefficient of determination values (R2) of the kinetics fitting data are listed in Table 3. The R2 values were found to be close to 1 by applying the second-order kinetics model and are much greater than that obtained by applying the first-order kinetics, Elovich, and Intra-particle diffusion models. This recommends that the second-order model is more satisfactory in describing the adsorption kinetics of As (III). The values of k2 and qe were estimated from the intercept and slope of the linear plot of t/qt versus t, respectively. The results are displayed in Fig. 9. It is clear that as the Sm dopant content increases from 0.01 to 0.08, the adsorption capacity increases. Furthermore, the highest adsorption rate was achieved in the presence of Mg0.33Ni0.33Co0.33SmxFe2-xO4 nanoparticles with x = 0.04.
Adsorption Isotherm
The adsorption performance between the As (III) and Mg0.33Ni0.33Co0.33Sm0.04Fe1.96O4 adsorbent can be determined by analyzing the adsorption isotherm. To do so, 40 mg of Mg0.33Ni0.33Co0.33Sm0.04Fe1.96O4 nanoparticles were mixed with As (III) solution (V = 50 mL, C= 5 mg.L-1 and pH = 2.69) at room temperature. Consequently, Langmuir, Temkin, and Freundlich isotherms were applied and the results are shown in Fig. 10. According to the Langmuir isotherm, each active site may only adsorb one molecule, and no interaction exists between adsorbate molecules occupying various active sites. The equation of the Langmuir isotherm is expressed as follows [39]:
where Ct (mg.L-1) signifies the As (III) concentration determined in the solution at any time t, qmax and qt (mg.g−1) is the maximum adsorption capacity and the adsorption capacity at any time t, and KL (L.mg-1) represents the Langmuir constant. The Temkin model, which considers the adsorbate-adsorbent interaction throughout the adsorption process, is expressed by the following equation [40]:
knowing that β is the coefficient linked to the sorption heat and KT is the Temkin equilibrium constant consistent with the maximum binding energy. Whereas, the Freundlich isotherm describes multilayer adsorption as well as interaction amongst the molecules that have been adsorbed. The linearized equation of this model is represented below [41]:
where n and KF symbolize the adsorption intensity and Freundlich constant, respectively. The negative slopes of the plots demonstrating Temkin and Freundlich isotherms (Fig. 10 (b)) indicate the failure of these isotherms in the description of the adsorption process of As (III) on Mg0.33Ni0.33Co0.33Sm0.04Fe1.96O4 nanoparticles. However, the linear plot denoting the Langmuir model, represented in Fig. 10 (a), shows the well-fitting of this model with the experimental adsorption data. Thus, qmax and KL, estimated from the slope and intercept of the Langmuir plot, are 5.05 mg.g-1 and 0.198 L.mg-1. As reported in a previous study, the removal of As (III) using copper II oxide nanoparticles followed the Langmuir model where qmax was 1.0862 mg.g-1 [42]. Thus, superior activity was revealed by Mg0.33Ni0.33Co0.33Sm0.04Fe1.96O4 nanoparticles in comparison to copper II oxide nanoparticles, as both exhibited conformity with the Langmuir isotherm.
Adsorption Performance of Nanoparticles for the Removal of Pb (II)
To evaluate the performance of Mg0.33Ni0.33Co0.33SmxFe2-xO4 nanoparticles with 0≤x≤0.08 for Pb (II) removal, the removal % of Pb (II) was estimated in the presence of 40 mg of each of the prepared nanoparticles after 120 min at room temperature. 50 mL of 5 mg.L-1 Pb (II) solution having a pH of 2.64 was used in this experiment. As shown in Fig. 11, the highest removal % is exhibited by Mg0.33Ni0.33Co0.33SmxFe2-xO4 nanoparticles with x = 0.01.
Effect of adsorbent amount
To investigate the effect of the adsorbent amount on the removal % of Pb (II), different masses of Mg0.33Ni0.33Co0.33Sm0.01Fe1.99O4 nanoparticles (20, 40, 60, and 80 mg) were mixed with 5 mg.L-1 Pb (II) solution and the removal % was calculated after 30, 60 and 90 min. The obtained results are shown in Fig. 12 (a). As the contact time between the Pb (II) solution and the adsorbent increases, the removal % also increases. In addition, the removal of Pb (II) is boosted in the presence of 80 mg of adsorbent compared to that in the presence of fewer amounts where 96 % of Pb (II) is removed after 90 min. Thus, increasing the amount of nanoparticles increases the removal of Pb (II). This is mainly attributed to additional adsorption sites acquired as the amount of nanoparticles increases.
Effect of Pb (II) initial concentration
The effect of Pb (II) initial concentration on the adsorption efficiency of Mg0.33Ni0.33Co0.33Sm0.01Fe1.99O4 nanoparticles was studied. Thus, 5, 7.5, and 10 mg.L-1 of Pb (II) solution was mixed with 40 mg of Mg0.33Ni0.33Co0.33Sm0.01Fe1.99O4 nanoparticles. As displayed in Fig. 12 (b), the removal % decreases with increasing the Pb initial concentration. The highest removal % was achieved in a 5 mg.L-1 Pb (II) solution. At low Pb (II) concentration, the availability of adsorption sites is relatively high. Thus, Pb (II) ions can be easily removed by adsorption. Whereas, as the Pb (II) concentration increases, the available adsorption sites of Mg0.33Ni0.33Co0.33Sm0.01Fe1.99O4 adsorbent are limited which in turn decreases the removal %.
CONCLUSION
Mg0.33Ni0.33Co0.33SmxFe2-xO4 nanoparticles were successively prepared by the co-precipitation method and used as adsorbents for the removal of heavy metal ions. XRD analysis reveals the purity and spinel structure of the prepared samples. The TEM image displays that the nanoparticles have a spherical morphology with an average particle size that ranges between 21.66 and 28.72 nm. An increase in the bandgap energy and a decrease in the Urbach energy was noticed upon Sm-doping. Doping nanoparticles with Sm improved the adsorption efficiency for the removal of As (III) and Pb (II). The adsorption of As (III) follows the second-order kinetics. Among the applied adsorption isotherms, the Langmuir model best fits the experimental data for the removal of As (III). Additionally, the optimum adsorbent amount of Mg0.33Ni0.33Co0.33Sm0.01Fe1.99O4 nanoparticles was 80 mg. As the Pb (II) concentration increases, the removal % decreases. This was owed to the reduction in the adsorption sites of Mg0.33Ni0.33Co0.33Sm0.01Fe1.99O4 nanoparticles at high Pb (II) concentration. The results restate the potential of ferrite nanoparticles for the removal of heavy metal ions from wastewater.
CONFLICT OF INTEREST
The authors hereby declare that there is no conflict of interest.
- Mahmud, H.N.M.E., A.O. Huq, and R. binti Yahya, The removal of heavy metal ions from wastewater/aqueous solution using polypyrrole-based adsorbents: a review. Rsc Advances, 2016. 6(18): p. 14778-14791.
https://doi.org/10.1039/C5RA24358K - Shokri, A. and K. Mahanpoor, Removal of ortho-toluidine from industrial wastewater by UV/TiO2 process. 2016.
- Shokri, A., M. Salimi, and T. Abmatin, Employing photo Fenton and UV/ZnO processes for removing Reactive red 195 from aqueous environment. Fresenius Environmental Bulletin, 2017. 26(2-A): p. 1560-1565.
- Qasem, N.A., R.H. Mohammed, and D.U. Lawal, Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean Water, 2021. 4(1): p. 36.
https://doi.org/10.1038/s41545-021-00127-0 - Kanwar, V.S., et al., Phytoremediation of toxic metals present in soil and water environment: a critical review. Environmental Science and Pollution Research, 2020. 27: p. 44835-44860.
https://doi.org/10.1007/s11356-020-11461-0 - Ince, M. and O.K. Ince, Heavy metal removal techniques using response surface methodology: water/wastewater treatment, in Biochemical Toxicology-Heavy Metals and Nanomaterials. 2019, IntechOpen.
https://doi.org/10.5772/intechopen.88915 - Peng, H. and J. Guo, Removal of chromium from wastewater by membrane filtration, chemical precipitation, ion exchange, adsorption electrocoagulation, electrochemical reduction, electrodialysis, electrodeionization, photocatalysis and nanotechnology: a review. Environmental Chemistry Letters, 2020. 18: p. 2055-2068.
https://doi.org/10.1007/s10311-020-01058-x - Tahir, M.B., H. Kiran, and T. Iqbal, The detoxification of heavy metals from aqueous environment using nano-photocatalysis approach: a review. Environmental Science and Pollution Research, 2019. 26(11): p. 10515-10528.
https://doi.org/10.1007/s11356-019-04547-x - Chai, W.S., et al., A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. Journal of Cleaner Production, 2021. 296: p. 126589.
https://doi.org/10.1016/j.jclepro.2021.126589 - Rajabi, M., et al., Comparison and interpretation of isotherm models for the adsorption of dyes, proteins, antibiotics, pesticides and heavy metal ions on different nanomaterials and non-nano materials-a comprehensive review. Journal of Nanostructure in Chemistry, 2023. 13(1): p. 43-65.
https://doi.org/10.1007/s40097-022-00509-x - Tamjidi, S., H. Esmaeili, and B.K. Moghadas, Application of magnetic adsorbents for removal of heavy metals from wastewater: a review study. Materials Research Express, 2019. 6(10): p. 102004.
https://doi.org/10.1088/2053-1591/ab3ffb - Jayalakshmi, R., J. Jeyanthi, and K.A. Sidhaarth, Versatile application of cobalt ferrite nanoparticles for the removal of heavy metals and dyes from aqueous solution. Environmental Nanotechnology, Monitoring & Management, 2022. 17: p. 100659.
https://doi.org/10.1016/j.enmm.2022.100659 - Shokri, A., Using NiFe2O4 as a nano photocatalyst for degradation of polyvinyl alcohol in synthetic wastewater. Environmental Challenges, 2021. 5: p. 100332.
https://doi.org/10.1016/j.envc.2021.100332 - Saghi, M., et al., The photo degradation of methyl red in aqueous solutions by α-Fe2O3/SiO2 nano photocatalyst. Journal of Nanoanalysis, 2018. 5(3): p. 163-170.
- Shokri, A., Photocatalytic degradation of nitrotoluene in synthetic wastewater by CoFe2O4/SiO2/TiO2 nanoparticles using Box-Behnken experimental design. DESALINATION AND WATER TREATMENT, 2022. 247: p. 92-99.
https://doi.org/10.5004/dwt.2022.28037 - Zahid, M., et al., Metal ferrites and their graphene-based nanocomposites: synthesis, characterization, and applications in wastewater treatment. Magnetic Nanostructures: Environmental and Agricultural Applications, 2019: p. 181-212.
https://doi.org/10.1007/978-3-030-16439-3_10 - Khoso, W.A., et al., Synthesis, characterization and heavy metal removal efficiency of nickel ferrite nanoparticles (NFN’s). Scientific Reports, 2021. 11(1): p. 3790.
https://doi.org/10.1038/s41598-021-83363-1 - Punia, P., et al., Adsorption of Cd and Cr ions from industrial wastewater using Ca doped Ni-Zn nanoferrites: synthesis, characterization and isotherm analysis. Ceramics International, 2022. 48(13): p. 18048-18056.
https://doi.org/10.1016/j.ceramint.2022.02.234 - Jadhav, S.A., et al., Rare earth-doped mixed Ni-Cu-Zn ferrites as an effective photocatalytic agent for active degradation of Rhodamine B dye. Journal of Rare Earths, 2023.
https://doi.org/10.1016/j.jre.2023.03.004 - Taneja, S., et al., Nanostructured Rare Earth Nd3+ doped Nickel-Zinc-Bismuth Spinel Ferrites: Structural, Electrical and Dielectric Studies. Ceramics International, 2022. 48(19): p. 27731-27738.
https://doi.org/10.1016/j.ceramint.2022.06.073 - Kirchberg, K., et al., Stabilization of monodisperse, phase-pure MgFe2O4 nanoparticles in aqueous and nonaqueous media and their photocatalytic behavior. The Journal of Physical Chemistry C, 2017. 121(48): p. 27126-27138.
https://doi.org/10.1021/acs.jpcc.7b08780 - Naik, C.C. and A. Salker, Structural, magnetic and dielectric properties of Dy3+ and Sm3+ substituted Co-Cu ferrite. Materials Research Express, 2019. 6(6): p. 066112.
https://doi.org/10.1088/2053-1591/ab0dd0 - Liu, Z., et al., Doping effect of Sm3+ on magnetic and dielectric properties of Ni-Zn ferrites. Ceramics International, 2017. 43(1): p. 1449-1454.
https://doi.org/10.1016/j.ceramint.2016.10.112 - Zhou, X., J. Wang, and D. Yao, Effect of rare earth doping on magnetic and dielectric properties of NiZnMn ferrites. Journal of Alloys and Compounds, 2023. 935: p. 167777.
https://doi.org/10.1016/j.jallcom.2022.167777 - Wu, X., et al., Effect of the rare-earth substitution on the structural, magnetic and adsorption properties in cobalt ferrite nanoparticles. Ceramics International, 2016. 42(3): p. 4246-4255.
https://doi.org/10.1016/j.ceramint.2015.11.100 - Hashim, M., et al., Influence of samarium doping on structural, elastic, magnetic, dielectric, and electrical properties of nanocrystalline cobalt ferrite. Applied Physics A, 2021. 127(7): p. 526.
https://doi.org/10.1007/s00339-021-04686-4 - Basma, H., et al., Enhancement of the magnetic and optical properties of Ni0. 5Zn0. 5Fe2O4 nanoparticles by ruthenium doping. Applied Physics A, 2022. 128(5): p. 409.
https://doi.org/10.1007/s00339-022-05552-7 - Chavan, P. and L. Naik, Investigation of energy band gap and conduction mechanism of magnesium substituted nickel ferrite nanoparticles. physica status solidi (a), 2017. 214(9): p. 1700077.
https://doi.org/10.1002/pssa.201700077 - Palaniappan, P., N. Lenin, and R. Uvarani, Copper substitution effect on the structural, electrical, and magnetic properties of manganese and lanthanum (Mn1− xCuxLa0. 1Fe1. 9O4) nanoferrites. Journal of Alloys and Compounds, 2022. 925: p. 166717.
https://doi.org/10.1016/j.jallcom.2022.166717 - Lemziouka, H., et al., Synthesis, structural, optical and dispersion parameters of La-doped spinel zinc ferrites ZnFe2-xLaxO4 (x= 0.00, 0.001, 0.005, 0.01 and 0.015). Vacuum, 2020. 182: p. 109780.
https://doi.org/10.1016/j.vacuum.2020.109780 - Ali, M., O. Hemeda, and A. Henaish, Tailoring changes in the physical, structural and linear optical properties of SrCuTi2Fe14O27 W-type hexaferrite-doped PVA polymeric composite films. Journal of Materials Science: Materials in Electronics, 2021. 32(12): p. 16038-16051.
https://doi.org/10.1007/s10854-021-06153-5 - Faramawy, A., et al., Structural, Optical, Magnetic and Electrical Properties of Sputtered ZnO and ZnO: Fe Thin Films: The Role of Deposition Power. Ceramics, 2022. 5(4): p. 1128-1153.
https://doi.org/10.3390/ceramics5040080 - Massoudi, J., et al., Magnetic and spectroscopic properties of Ni-Zn-Al ferrite spinel: from the nanoscale to microscale. RSC advances, 2020. 10(57): p. 34556-34580.
https://doi.org/10.1039/D0RA05522K - Tanbir, K., et al., Effect of doping different rare earth ions on microstructural, optical, and magnetic properties of nickel-cobalt ferrite nanoparticles. Journal of Materials Science: Materials in Electronics, 2020. 31: p. 435-443.
https://doi.org/10.1007/s10854-019-02546-9 - Chowdhury, S.R. and E.K. Yanful, Arsenic and chromium removal by mixed magnetite-maghemite nanoparticles and the effect of phosphate on removal. Journal of environmental management, 2010. 91(11): p. 2238-2247.
https://doi.org/10.1016/j.jenvman.2010.06.003 - Zhao, B., et al., Preparation of acrylamide/acrylic acid cellulose hydrogels for the adsorption of heavy metal ions. Carbohydrate polymers, 2019. 224: p. 115022.
https://doi.org/10.1016/j.carbpol.2019.115022 - Wekoye, J.N., et al., Kinetic and equilibrium studies of Congo red dye adsorption on cabbage waste powder. Environmental Chemistry and Ecotoxicology, 2020. 2: p. 24-31.
https://doi.org/10.1016/j.enceco.2020.01.004 - Masinga, T., M. Moyo, and V.E. Pakade, Removal of hexavalent chromium by polyethyleneimine impregnated activated carbon: Intra-particle diffusion, kinetics and isotherms. Journal of materials research and technology, 2022. 18: p. 1333-1344.
https://doi.org/10.1016/j.jmrt.2022.03.062 - Matouq, M., et al., The adsorption kinetics and modeling for heavy metals removal from wastewater by Moringa pods. Journal of Environmental Chemical Engineering, 2015. 3(2): p. 775-784.
https://doi.org/10.1016/j.jece.2015.03.027 - Umeh, T.C., J.K. Nduka, and K.G. Akpomie, Kinetics and isotherm modeling of Pb (II) and Cd (II) sequestration from polluted water onto tropical ultisol obtained from Enugu Nigeria. Applied Water Science, 2021. 11(4): p. 1-8.
https://doi.org/10.1007/s13201-021-01402-8 - Naseem, K., et al., Adsorptive removal of heavy metal ions using polystyrene-poly (N-isopropylmethacrylamide-acrylic acid) core/shell gel particles: adsorption isotherms and kinetic study. Journal of Molecular Liquids, 2019. 277: p. 522-531.
https://doi.org/10.1016/j.molliq.2018.12.054 - Goswami, A., P. Raul, and M. Purkait, Arsenic adsorption using copper (II) oxide nanoparticles. Chemical engineering research and design, 2012. 90(9): p. 1387-1396.
https://doi.org/10.1016/j.cherd.2011.12.006