Document Type : Original Research Paper
Authors
1 Symbiosis International (Deemed University), Symbiosis Institute of Technology, Lavale, Pune-412115, India
2 Department of Applied Sciences, COEP Technological University, Pune-411005, India
Abstract
The present study features the synthesis of Dodecylbenzene sulphonic acid-doped polypyrrole tungsten oxide (PPy-WO3) nanocomposites and its photocatalyic studies on Methylene Blue (MB). The nanocomposites of PPy with a very low concentration of WO3 were prepared using 0.05 to 0.3 wt.%, of WO3 nanoparticles. The composites were characterized by using-ray Diffraction, FTIR and FESEM for phase identification, morphological studies. The electrical conduction of the nanostructured materials at room temperature exceeded that of PPy, with the electrical conductivity increasing linearly with higher WO3 concentrations. The band gap for the nanocomposite was found to be 2.12eV. The PL spectra substantiated the adequate segregation of charge carriers photoexcited in the samples. The nanocomposites were tested as photocatalysts for the degradation of methylene blue dye (MB). A two-step mechanism has been propsed for dye removal: adsorption (in the absence of UV light) and photodegradation on the photocatalyst surface (in the presence of UV light). The maximum removal efficiency for methylene blue dye was 98.31% in UV light for 0.16 g/L of the 0.3 wt.% PPy- WO3 nanocomposite, with a dye concentration of 5 mg/L. The addition of p-benzoquinone (*O2- scavenger) contributed to a significant decrease in the photodegradation efficiency of the catalyst, i.e.,63.21%, and thus can be believed as the main active species for the degradation of the methylene blue dye.
Graphical Abstract
Keywords
INTRODUCTION
The advancements in photocatalysis offer promising prospects for the large-scale purification of contaminated water and air. [1]. As the demand for environmentally friendly solutions grows, significant research focuses on developing solar-responsive photocatalysts to efficiently degrade various wastewater contaminants under light irradiation [2]. The textile industry is one of the biggest global polluters. The increased usage of dyes and the discharge of untreated effluents into the water bodies, which mainly contain industrial pollutants and dyes, lead to significant environmental damage caused by the textile industries [3]. Due to challenges with dyeing, a sizeable part of dyestuff is lost directly to wastewater during the manufacture of textiles, which ultimately makes its way into water bodies. The transformation of dangerous organic pollutants such as dyes into specific, nontoxic elements is given much attention because most colorants exhibit significant toxicity and possess non-degradable characteristics [4,5]. One such pollutant, Methylene Blue (MB) (3,7 -bis (Dimethyl amino)-phenothiazine-5-mum chloride), a cationic dye, is primarily employed in the textile sector to tone down the colors of silk and to color paper [6] It is used for healthcare as a medicine for several therapeutic and screening procedures. Due to its hydrophilic characteristics, intricate aromatic compounds, and exceptional resistance to temperature, light, and chemical changes, it cannot be broken down using conventional water treatment techniques, damaging the ecosystem. Several water treatment techniques remove these contaminants from water streams, including membrane separation, coagulation, adsorption, and distillation [6,7]. Among these methodologies, adsorption has been established as the most competitive technique due to its economical nature, uncomplicated design, effortless operation, minimal generation of unsafe residues, the accessibility of a broad range of absorptive materials, and convenient recovery of said materials [8, 9]. However, a notable challenge in these approaches is reaching adsorbent saturation, which requires either regeneration or substitution. These factors can concurrently impose additional burdens on process costs and post-process treatments. Conversely, photocatalysis can decompose particles that present a challenge for complete removal through conventional techniques in water treatment [10]. The strong adsorption of contaminants onto the adsorbent is subsequently followed by their migration to the surface of the photocatalyst, leading to an extensive photodegradation process, as evidenced by research. [11].
Photocatalytic degradation has garnered significant attention for removing dyes due to its environmentally friendly and effective characteristics. The photodegradation route involves the rupture of dye molecules, forming simpler organic compounds that eventually undergo total mineralization [12–16]. However, numerous photocatalysts exhibit limited adsorption capacity for organic pollutants, and active species like photogenerated holes and certain free radicals play a restricted role [17]. These drawbacks can be addressed by integrating adsorption and photocatalysis to eliminate organic dyes from wastewater. The synergistic approach of combining adsorptive and photocatalytic processes constitutes a novel and emerging technology in water pollution management [18]. Low-concentration pollutants in water are initially adsorbed and concentrated within the structure and then undergo in-situ oxidation through the photocatalytic process. Sequential adsorption and photocatalytic degradation enable the degradation of adsorbed organic dyes within a shorter timeframe, thereby significantly extending the lifespan of the adsorbent. This approach also resolves the saturation issue associated with the adsorption method and the separation problem encountered in photocatalytic degradation [19-21]. In our study, we have reported combined adsorption and photocatalytic degradation to remove Methylene Blue dye effectively.
Conductive polymers have garnered considerable interest over the past thirty years as innovative materials owing to their industrial importance, exceptional durability in various environmental contexts, electrical conductance, and remarkable mechanical, optical, and electronic characteristics [22]. It is also interesting to note that conjugated polymer nanostructures have recently been shown to be highly active for air and water treatment, including water splitting, under UV and visible light [23]. Various categories of conductive polymers, including polyaniline (PANI), polyacetylene (PA), poly(p-phenylenevinylene) (PPV), polypyrrole (PPy), poly-furan (PF), poly (3,4-ethylene dioxythiophene) (PEDOT), and various modifications of polythiophene (PTh), have gained considerable interest in the realm of nanotechnology owing to their unique and outstanding characteristics. These attributes encompass electrical characteristics, a reversible process for conduction, a dopant/undopant process that can be tuned, adjustable chemical and electrochemical properties, and simplicity of processing [24,25]. Amongst the various existing polymers, Polypyrrole (PPy) has been extensively studied due to its unique features, such as the ability for versatile fabrication, comparatively elevated electrical conductance, and remarkable mechanical robustness [26]. Polypyrrole and its composites have been reported to be a promising adsorbent and a photocatalyst for the degradation of waste contaminants in water, especially organic dyes, due to its aromatic structure and presence of nitrogen atoms which are likely to be responsible for its interaction with dyes [27].
Semiconductor oxides, such as titanium dioxide (TiO2), zinc oxide (ZnO), tin dioxide (SnO2), and tungsten trioxide (WO3), have garnered the attention of researchers due to their remarkable ability to break down organic compounds [28,29]. This is primarily attributed to their exceptional photocatalytic efficiency when incorporated into the polypyrrole host matrix. Moreover, these oxides possess excellent biocompatibility, are competitively priced, exhibit superior chemical and physical properties, and possess a potent capacity to convert harmful organic contaminants into benign substances like carbon dioxide (CO2) and water (H2O). Polypyrrole as a host framework facilitates optimal electron transfer within the visible spectrum [26,29]. The bandgap of WO3, an n-type semiconductor, is approximately 2.7 electron volts (eV). WO3 is commonly used as a photocatalyst due to its ability to absorb light. However, its conduction band is relatively low, making it unsuitable for hydrogen production and limiting its applications. The valence and conduction bands of WO3 are situated at energy levels of 0.74 eV (electron conduction band, ECB) and 3.4 eV (electron valence band, EVB), respectively. [30,31]. It is widely recognized that organic contaminants undergo degradation through photocatalytic processes when they come in contact with semiconductors in the presence of ultraviolet and visible light. This leads to the formation of electron-hole pairs, with excited electrons from the valence band moving to the conduction band, creating holes that form hydroxyl radicals and superoxide ions, which effectively break down toxic compounds into safe byproducts [29].
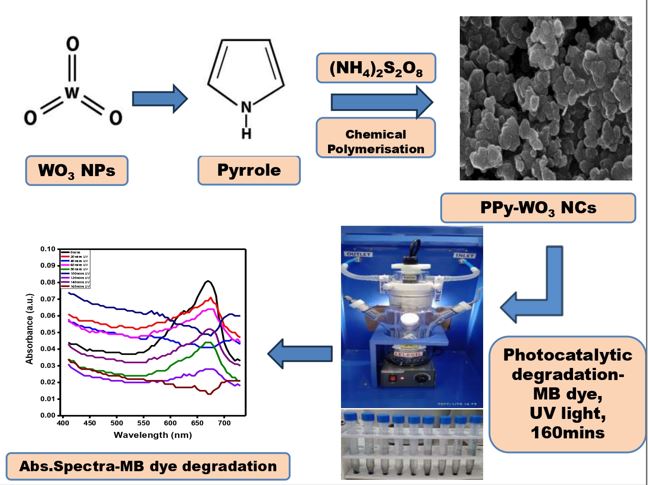
In this research, to the best of the researcher’s knowledge, we present the fabrication of nanocomposites of polypyrrole by adding a very low concentration of nanosized WO3, ranging from 0.05 wt. % to 0.3 wt. %. This demonstrates the feasibility of producing conducting polymers for photodegradation applications using an economical and environmentally friendly method. Our investigation involved the addition of minute concentrations of tungsten oxide nanoparticles to the polypyrrole matrix, resulting in the formation of hybrid nanocomposites. An in-situ chemical polymerization technique was employed, incorporating a small quantity of tungsten oxide nanomaterials into the polypyrrole network with the aid of the dopant 4-Dodecyl benzene sulfonic acid. The present study highlights the results of PPy-WO3 hybrid nanostructured materials evaluated as photocatalysts for degrading Methylene Blue dye using a two-step mechanism of both adsorption and photocatalytic degradation under ultraviolet irradiation.
EXPERIMENTAL DETAILS
Chemicals used.
Pyrrole was obtained from Kemphasol, World of Chemicals India. Tungsten (VI) Oxide nanoparticles with a size below 100 nm were acquired from Aldrich Chemistry, India. 4-Dodecyl Benzene Sulphonic Acid and Ammonium persulfate were procured from Aldrich Chemistry, India, and Fischer Scientific, India. Methylene Blue dye (C16H18ClN3S) obtained from Loba was utilized for photocatalytic investigations.
Synthesis of Polypyrrole-Tungsten Oxide Hybrid Nanocomposites
Polypyrrole-tungsten oxide hybrid nanocomposites (PPy-WO3) were prepared through an in-situ synthetic crosslinking technique employing Pyrrole (Py) and Ammonium Persulphate (APS) in a molar ratio of 1:1.5M and 0.05 M 4-Dodecyl benzene sulphonic acid (DBSA). WO3 nanoparticles, measuring less than 100 nm in size, were used at varying concentrations of 0.05, 0.1, 0.2, and 0.3 wt.%. The WO3 nanomaterials were introduced to 0.05M 4-dodecyl benzene sulphonic acid prepared in deionized water and subjected to magnetic stirring for 15 minutes to ensure homogeneous mixing. A solution containing pyrrole at a concentration of 1M dissolved in ethanol was added gradually, drop by drop, to a well-mixed combination of WO3 nanomaterials and DBSA at a temperature of 0°C while stirring continuously. The addition process was completed within 15 minutes. Afterwards, a 1.5 M aqueous solution of APS was slowly added while stirring continuously, taking 30 minutes to complete the addition. The entire procedure was conducted at 0°C, and the reaction time was varied at intervals of 4 hours, 8 hours, 12 hours, and 16 hours. Following forming a black solid precipitate, it underwent multiple rounds of filtration using ethanol and deionized water to remove any oligomers and potential impurities. The resulting precipitate was then air-dried for 24 hours at room temperature. To obtain finely powdered PPy, the black solid powder obtained was ground using an agate pestle and mortar. Subsequently, the PPy powder underwent further characterization.Fig. 1(a). represents the reaction mechanism of the DBSA-doped PPy-WO3 nanocomposite.
Photocatalytic Degradation Studies
The photodegradation of Methylene Blue dye (MB) was carried out employing a photocatalytic device produced by Lelesil Innovative Systems (LIS-5D model). The photocatalytic device comprises a 500 ml glass assembly with three arms. One arm to collect the sample, the second to measure the temperature with the help of a thermocouple, and the third to load the catalyst. The irradiation was carried out using a 250W UV light source mounted on a double-layered quartz enclosure equipped with water circulation systems to maintain the temperature below 250C using ice cubes.
PPy, WO3, PPy-WO3 nanocomposites of varying amounts, i.e., 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4, 1.6 and 1.8 g/L were dispersed in 100 ml of 5 mg/l of MB dye solution. This mixture was then stirred in the dark for 20 minutes. A specific sample volume was withdrawn using a syringe at a time interval of 20 minutes until 160 minutes had passed. The sample was centrifuged to separate the photocatalyst from the dye solution. The degradation effectiveness of the MB dye was assessed utilizing an ultraviolet-visible absorption spectrophotometer (Equiptronics, EQ826 model). The measurement of absorbance at the peak wavelength of MB, specifically 665nm, was recorded. The same experiment was carried out by varying the pH of the solution. A similar adsorption study was conducted in the dark, varying the identical amounts of catalysts (0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4, 1.6 and 1.8 g/L) dispersed in 100ml of 5mg/L of MB dye solution at pH 7 and the time of contact to 160mins. The adsorption efficiency of the MB dye was measured by recording the absorbance at the maximum wavelength of MB, i.e.,665nm.
The pH studies by varying the pH between 4,7 and 9 were carried out using 1.6 g/L PPy, WO3, and PPy-WO3 nanocomposite, and the effect of visible light on adsorption and photocatalytic degradation was carried out using 1.6 g/L PPy-WO3 nanocomposite as it showed good results.
Exposure to visible light was carried out using 1.6 g/L PPy, WO3, and PPy- WO3 nanocomposite. A 250W Visible light source was used to carry out the experimentation.
The adsorption and photocatalytic degradation efficacy of the MB dye was calculated using Eq. 1
Eq. 1
Co is the initial concentration of the dye in parts per million before irradiation with UV light, and Ct is the dye concentration in parts per million after irradiation at a time ‘t’.
CHARACTERIZATION
The Fourier transform infrared spectroscopy (FT-IR) technique used the Shimadzu-IR affinity 1S model to examine the PPy, WO3, and PPy-WO3 hybrid composites. X-ray diffraction (XRD) experiments were conducted using the model, Rigaku Miniplex 600. The X-ray Diffraction spectra were obtained using Cu K radiation (k=1.5406) over a 2θ range of 10-80°. The XRD spectra were analyzed by comparing the detected peaks in correlation with the standard pattern, from the JCPDS file. The surface characteristics of PPy and PPy-WO3 hybrid nanocomposites were investigated using Field-emission SEM provided by Carl Zeiss India Ltd., model, Sigma IV. The Field-emission SEM images were utilized to determine the grain size. Conductivity measurements were performed using the Keithley 2000 instrument with two probes.
RESULTS AND DISCUSSION
Structural Studies
Fig. 1. illustrates the X-ray diffraction patterns of PPy and PPy-WO3 hybrid nanostructured materials with varied amounts of WO3 nanomaterials (0.05, 0.1, 0.2, and 0.3 wt.%). The X-ray diffraction spectra of Polypyrrole exhibits a broad hump at 22°. The XRD spectra of tungsten oxide associate precisely with the orthorhombic phase of WO3, as indicated by the matching Joint Committee on Powder X-ray Diffraction Standards (JCPDS) file No. 71-0131 [32].
The X-ray Diffraction spectrum of the PPy-WO3 nanocomposites (0.05, 0.1, 0.2, and 0.3 wt.%) is presented in Fig. 1(b). display the characteristic diffraction peaks of WO3, along with the broad hump at 22°, corresponding to PPy. The intensity of the PPy hump decreases as the concentration of WO3 nanomaterials increases. All the XRD spectra of PPy-WO3 nanocomposites exhibit prominent peaks originating from the (002), (020), (220), and (200) planes, which are indicative of the crystal lattice structure of WO3 integrated within the PPy polymer chains. These results are consistent with the crystal structure of WO3 described in JCPDS No. 71-0131[33-36].
The lack of foreign matter peaks indicates the successful production of a highly pure PPy-WO3 hybrid nanocomposite. The characteristic diffracted pattern of WO3 nanoparticles is visible in the PPy-WO3 hybrid nanocomposite, suggesting that the crystallization of WO3 nanoparticles is unaffected by the presence of PPy, and no additional ordered arrangement has been incorporated into the hybrid nanocomposites. The broad intensity peak of polypyrrole overlaps with the crystal planes of WO3 nanomaterials, specifically the (0 0 2), (0 2 0), and (2 0 0) planes, which exhibit strong diffraction intensities. Furthermore, an increase in the concentration of WO3 nanomaterials in the polymer-based composite results in a sharper and more intense diffraction peak of PPy, indicating that the proportion of metal oxide has an impact on the molecular arrangement of the hybridized composite, making the hybridized composites more crystalline compared to pure PPy. The peak widths remain relatively constant, suggesting a homogeneous distribution of WO3 nanoparticles throughout the PPy framework without significant agglomeration [33-36]
The Scherrer equation was applied to the XRD spectra to determine the dimensions of the crystalline particles. The size of the crystalline particles was computed using the equation given in Eq. 2, and the results are presented in Table 1.
Eq. 2
The variable D represents the mean size of the crystalline particles in nanometres, λ represents the wavelength of the X-ray from the Cu Kα source (0.15418 nm), β corresponds to the angular width of the X-ray diffraction peak at half maximum (FWHM) in radians, and θ denotes the angle of Bragg’s law in degrees. Table 1. displays the calculated average values for the size of the crystalline particles. The average crystallite size is observed to decrease as the concentration of WO3 nanoparticles increases from 0.05 to 0.3 wt%. The average crystallite size calculated for the PPy-WO3 (0.3wt%) nanocomposite is 3.4nm. The decrease in the size is advantageous for achieving a high surface-to-volume ratio in PPy-WO3(0.3 wt.%) nanocomposites, making them suitable for photocatalytic applications.
Proposed Mechanism of Reaction
The proposed mechanism for the formation of doped polypyrrole tungsten oxide nanocomposite is that dodecylbenzene sulphonic acid forms salt with pyrrole through an acid-base reaction while it is being polymerized in the presence of ammonium persulfate. This, in the presence of Tungsten oxide nanoparticles, forms a complex as shown in Fig. 1(a).
FESEM
Fig. 2. illustrates the field-emission scanning electron microscopy (FE-SEM) images of Dodecyl Benzene Sulphonic Acid (DBSA) doped Polypyrrole (PPy) and Polypyrrole-Tungsten trioxide (PPy-WO3) (0.05, 0.1, 0.2, and 0.3 wt.%) composite materials under investigation. The surface topography of the DBSA doped PPy-WO3 (0.05, 0.1, 0.2, and 0.3 wt.%) hybrid composite exhibits predominantly interconnected spherical particles visible as compact agglomerates, which become more pronounced with increasing concentrations of WO3, as depicted in Fig. 2(e). No evidence of any secondary phase formation or significantly different polymer or oxide particles was observed, indicating the homogeneous distribution of the WO3 nanoparticles in the PPy matrix [33-36].
Grain Size
The determination of grain size was performed based on the field-emission scanning electron microscopy (FE-SEM) images shown in Fig. 3. The analysis revealed a reduction in grain size from 151.11 nm to 95.88 nm as the concentration of WO3 increased from 0 wt.% to 0.3 wt.%. This trend indicates that higher concentrations of WO3 result in a decrease in grain size. Such a reduction in grain size is advantageous for achieving an elevated ratio of surface area to volume in PPy-WO3 conducting polymers, making them suitable for photodegradation applications [24].
FTIR analysis
The Fourier Transform Infrared spectrum of DBSA doped polypyrrole and PPy-Tungsten trioxide (WO3) nanocomposites at various concentrations (0.05, 0.1, 0.2, and 0.3 wt.%) are depicted in Fig. 4. A noticeable shift in the peak positions is observed in the FTIR spectra of the PPy- WO3 nanocomposite samples, as indicated in Table 2. This shift suggests a decrease in molecular arrangement and connectivity resulting from the modifications in PPy by including DBSA and WO3. The chemical reactions among PPy, DBSA, and WO3 may induce alterations in the functional positions of PPy. Therefore, the FTIR analysis provides evidence for forming nanocomposites comprising DBSA-doped PPy and WO3 [33-35].
UV-Vis Diffuse Reflectance Spectra
UV-visible spectroscopic investigations were conducted to scrutinize the optical properties of Polypyrrole (PPy), Tungsten oxide (WO3), and PPy-WO3 nanocomposites with a 0.3wt% loading (Fig.5(d)). The Tau’c plot methodology was employed to ascertain the band gap of all these materials, namely, WO3, PPy, and PPy-WO3 nanocomposites with 0.3wt% content (Fig.5(a, b, c)). The Tau’c technique is delineated by Eq. 3:
Eq. 3
In this equation, α signifies the absorption coefficient (α = 2.303A/t), where A represents the absorbance, and t stands for the thickness of the cuvette. Other variables encompass h (Planck’s constant), ν (photon frequency), Eg (optical bandgap), and n, which assumes values of ½, 2, 3/2, and 3 corresponding to direct allowed, indirect allowed, direct forbidden, and indirect forbidden transitions, respectively.
When the linear segment of (Ahν)2 is extended along the x-axis in a plot of (Ahν)2 versus hν, it furnishes the optical bandgap value (refer to Fig. 5(a, b, c)). In this instance, the calculated optical bandgap values were determined to be 2.43 eV, 2.27 eV, and 2.12 eV, correspondingly [55].
The Eg (optical bandgap) decreases with the introduction of WO3 doping into the PPy structure, indicative of an enhanced charge transfer occurring on the surface in association with the WO3 d-d transition [56]. Upon incorporation of WO3 nanoparticles into the PPy polymer matrix, a novel energy state emerges within the energy gap of PPy-WO3, consequently leading to a reduction in the Eg value of the composite [57].
Photoluminescence Spectra
The photoluminescence (PL) spectra of the specimens depicted in Fig. 6 were acquired at room temperature using an excitation wavelength of 350 nanometers (nm). The PL spectra substantiate the adequate segregation of charge carriers photoexcited in the samples. This method is valuable for investigating photo-excited charge carriers’ transfer and recombination characteristics.
Four emission peaks were evident in the PL spectra, centered at 377 nm, 396 nm, 451 nm, and 468 nm, and a faint band at 492 nm was discernible in all three samples presented in Fig. 6 (refer to the inset graph). Within these spectra, the 377 nm and 396 nm bands denote blue emission within the UV range, commonly called near band edge (NBE) emission, stemming from the emission of free excitons [60]. The 468 nm and 492 nm bands indicate a blue-green emission due to structural defects and impurities [61, 62]. The prominent shoulder peak at 451 nm is ascribed to the indirect band-to-band transition and the introduction of charge-carrier trapping states induced by surface oxygen vacancies [63].
The composite’s intensity of PL emission diminished, indicating that the integration of WO3 in PPy effectively curtailed the charge recombination process, attributable to an oxygen vacancy layer at the interface. Additionally, the oxidative species (polaron and bipolaron) of PPy further entrap the charge carriers, thereby elongating the lifespan of generated excitons, ultimately leading to an augmented photoactivity [55, 63].
Electrical Conductivity Studies
Fig. 7 illustrates the changes in ambient temperature conductivity of DBSA-doped PPy- WO3 (0–0.3 wt. %) hybrid nanocomposites. It can be observed from Fig. 7 and Table 3 that the room temperature direct current (DC) conductivity of the hybrid nanocomposite increases as the content of WO3 nanoparticles in the PPy matrix increases. PPy exhibits a room temperature conductivity of 5.23 × 10⁻7 S/cm. The PPy matrix with the highest concentration of WO3 nanoparticles (0.3 wt. %) demonstrates a conductivity of 3.89× 10⁻6 S/cm. The observed increase in direct current (DC) electrical conductivity in the PPy-WO3 (0.05-0.3 wt. %) hybrid nanocomposites can be attributed to enhanced electron mobility, elongation of polymer chains and an augmented probability of electron tunneling in all subsequent composite materials. This behavior can be attributed to the versatile semiconducting properties of WO3. In this context, a rise in the concentration of semiconducting tungsten oxide nanoparticles leads to improved conjugation and enhanced compactness, resulting in increased conductivity of PPy–WO3 hybrid nanocomposites. The WO3 nanoparticles likely serve as anchor points or cross-linkers for the polymer chains. When there are more WO3 nanoparticles, they can form stronger connections between the polymer chains. This improved conjugation enhances the overall structural integrity and stability of the nanocomposite. Also, the nanoparticles might fill in gaps and voids in the polymer matrix, leading to a more compact and uniform structure. The DC conductivity of PPy is low as compared to the 0.3wt% composite but higher than what was reported by Mane and his colleagues [33] for pure PPy due to the addition of DBSA. Due to its anionic nature, DBSA (dodecylbenzene sulfonic acid) can act as a surfactant, establishing a conductive pathway within the PPy–WO3 nanostructured composites and preventing the clustering of active materials. Consequently, the electrical conductivity of PPy–WO3 nanocomposites at room temperature increases. Additionally, in the PPy–WO3 nanostructures composites, polarons or bipolarons form because of the delocalization effect during the doping process, thereby increasing the electrical conductivity of PPy–WO3 nanocomposites. This can be attributed to the ionic dopant DBSA, which typically introduces protons into the nitrogen-binding sites within the polypyrrole framework, thereby augmenting the number of charge carriers, which leads to an elevation in electrical conductivity [35].
Adsorption and Photocatalytic Studies-The Effect of Operating Parameters on the Adsorption and Photocatalytic degradation of Methylene Blue Dye
The adsorption and photocatalytic degradation of Methylene Blue Dye was carried out under two different conditions: in the dark and the presence of UV light. The operating parameters, such as catalyst loading, pH, and contact time, were varied.
Effect of contact time between the MB dye and the type of catalyst (PPy, WO3 and PPy- WO3 nanocomposites)
The influence of different exposure durations to UV light on methylene blue (MB) dye in the presence of varying concentrations of the photocatalysts, namely doped Polypyrrole (PPy), Tungsten trioxide (WO3), and doped PPy-WO3 composite, was examined. Similarly, the impact of time on adsorption processes conducted in darkness was investigated using the same types and concentrations of photocatalysts. The concentration of photocatalysts for degrading methylene blue ranged from 0.2 g/L to 1.8 g/L, while the treatment time was maintained at 160 minutes in both studies. The dye concentration was constant at five parts per million (ppm).
Fig. 9 (a) illustrates the optical absorption profiles, depicting the combined adsorption and photodegradation of MB dye using PPy-WO3 nanocatalysts (1.6 g/L) under UV light irradiation. The characteristic peak wavelength’s intensity diminishes with UV light exposure, and the inset image demonstrates a gradual reduction in the supernatant’s color intensity, indicating decolorization [36].
As shown in Fig. 9 (b), the adsorption of MB dye onto the PPy-WO3 catalyst reaches 78.48% after 20 minutes of stirring in the dark. On exposure to UV radiation for 160 minutes at a catalyst loading of 1.6 g/L and pH 7, photocatalytic degradation achieves a maximum of 98.13%. The adsorption of the MB dye by conducting adsorption studies under similar conditions (0.2g/L to 1.8g/L photocatalysts PPy, WO3, and PPy-WO3,160mins contact time, pH 7) in the dark was observed to be 88.86%. (Fig. 9(d)). It indicates the compelling synergy between adsorption and photocatalytic degradation in removing the MB dye.
The interaction duration between the dye and the PPy-WO3 catalyst (0.3 wt.%) is a critical parameter determining the saturation point for MB dye adsorption on the catalyst’s surface. The characteristics of the dye and the catalyst influence this duration. Under optimal conditions, the suspension was initially stirred in the dark (adsorption) to determine the maximum dye adsorption on the catalyst. Results revealed that within 160 minutes of dark agitation, approximately 88.86% of the dye was adsorbed on the catalyst. Under similar conditions, when the suspension was stirred in the dark (adsorption) for 20 minutes, approximately 78.48% of the dye was adsorbed. Upon 160 more minutes of continuous agitation with exposure to UV light, the final dye removal reached around 98.13%.
This phenomenon can be explained by the fact that the efficiency of dye adsorption on the catalyst increases until sufficient active adsorption sites are available. The remaining semiconductor sites, which might not be involved in adsorption, contribute to the photocatalytic degradation of MB under UV light through electron transfer from the valence band to the conduction band. In photoluminescence investigations with UV light, the MB removal efficiency increased linearly. These results suggest the simultaneous adsorption and photocatalytic degradation of MB dye molecules on the catalyst surfaces [37].
The combined efficiency of adsorption and photodegradation and the sole adsorption capacity of MB on the PPy photocatalyst were 94.82% and 90.24%, respectively (Fig. 8 (b&d). For WO3, these values were observed to be 93.11% and 76.86%, respectively (Fig. 8 (f&h)). Fig. 10 (a-c) depicts the effect of contact time and type of catalyst for the combined adsorption and photocatalytic degradation of the dye and dye removal due to adsorption. This very clearly explains the efficiency of the PPy-WO3 (0.3 wt.%) nanocomposite as both an effective adsorbent and photocatalyst for MB dye degradation.
Effect of type of Catalyst and Catalyst Loading
To determine the most effective concentration of photocatalysts for degrading methylene blue, the impact of catalyst dosage on dye adsorption and degradation efficiency was investigated by changing the concentration of the catalyst from 0.2 g/L to 1.8 g/L while keeping the concentration of the dye constant at five ppm(5ppm) (Fig. 11). The findings revealed a significant influence of photocatalyst dosage on the photodegradation of MB dye. It was observed that increasing the catalyst loading enhances the efficacy of degradation, but beyond a certain threshold, the effectiveness starts to decline [38,39]. By increasing the catalyst load to an appropriate level, more active sites are available, promoting the generation of electron-hole pairs, *OH radicals, and O2 - ions, which play a crucial role in enhancing the efficacy of photocatalytic degradation. Conversely, exceeding a specific limit in catalyst load leads to particle-particle interactions, resulting in a screening effect that hampers photon accessibility to the photocatalyst interface, thereby decreasing the efficacy of photodegradation [7,40,41].
Fig. 11. present results for the adsorption and degradation experiments conducted with 5 ppm of MB and catalyst loads of 0.2 to 1.8 g/L for the catalysts PPy-WO3 (0.3wt%) nanocomposite, PPy, and WO3. The respective adsorption and degradation efficiencies for PPy-WO3 (0.3wt%) nanocomposite for catalyst load of 0.2g/L,1.0g/L, 1.6g/L and 1.8g/L were found to be 46.37 %, 89.13 %, 98.13% and 93.53% for methylene blue which is higher than the adsorption and degradation efficiencies of the catalysts PPy and WO3.
Effect of pH
Typically, industrial effluents exhibit a wide range of pH values. Since the pH of industrial effluent is not constant, it is crucial to investigate the pH at which it degrades most rapidly. Moreover, the solution’s pH is a contributing factor that influences both the dye adsorption and degradation rate and the production of hydroxyl radicals [7]. The adsorption and photodegradation experiments were conducted at three distinct pH levels: 4, 7, and 9, by varying the type of catalyst: PPy-WO3, PPy, and WO3 while maintaining a constant catalyst load of 1.6 g/L and a consistent dye concentration of 5 ppm. The catalyst load, which showed the maximum degradation, was considered for pH studies. The results demonstrate that as the pH increased from 4 to 7, the adsorption and degradation were higher, and on further increase in the pH to 9, the adsorption and degradation of the MB dye decreased. Also, the combined adsorption and photodegradation values for the nanocomposite PPy-WO3 were recorded to be more than PPy and WO3, respectively. At a pH of 4, the adsorption and degradation rate of MB dye with PPy-WO3 (0.3wt %) nanocomposite reached approximately 87.54% after 160 minutes. In neutral pH conditions (pH 7), about 98.31% of the dye underwent degradation, while at pH 9.0, the degradation reached approximately 87% within 160 minutes. At a pH of 4, the adsorption and degradation rate of MB dye with PPy and WO3 reached approximately 82.53% and 74.19% after 160 minutes. In neutral pH conditions (pH 7), about 88.86% and 93.17% of the dye was degraded, while at pH 9.0, the degradation reached approximately 74.19% and 87% within 160 minutes (Fig. 12, Table 5).
The probable reaction mechanism between the MB dye and the composite material at the pH of 4,7 & 9 is explained below.
The pH of the solution influences the adsorption and photocatalytic degradation of dyes from wastewater. Therefore, this parameter is extensively investigated to achieve maximum adsorption efficiency by determining the optimal pH value for the degradation process. This change in adsorption and photocatalytic degradation behavior can be ascribed to alterations in the surface charge of the adsorbent and dye molecules. At pH 4, when the system is highly protonated, the PPy-WO3 carries a positive charge as the lone pair of electrons of the free Nitrogen on the Pyrrole group is fully utilized in bonding with the H+ ions. Therefore, MB adhesion is lost primarily due to the electrostatic repulsion between the dye and photocatalyst. A significant reduction in dye adsorption in alkaline media can be attributed to electrostatic repulsion between the negatively charged adsorbent and deprotonated dye particles. In addition to electrostatic interactions, hydrogen bonding, and hydrophobic-hydrophobic reactions may also play significant roles in governing and controlling adsorption [50-52].
Effect of Visible Light
The photocatalytic degradation of Methylene blue dye using 250W Visible light was carried out using 1.6g/L PPy-WO3 nanocomposite at pH 7, and the duration of contact time was maintained at 160mins. Fig. 13 (a) represents the absorption spectra, and Fig. 13 (b) represents the effect of contact time for combined adsorption and photocatalytic degradation of Methylene blue dye. The adsorption in the dark after 20 mins was found to be 61.16%, and on further exposure to visible light for 160 mins, the photocatalytic degradation was found to be 73.78%. This is less as compared to the degradation, which was found using UV light.
Photodegradation Reaction Mechanism
The experiments have contributed to a deeper understanding of the photocatalytic mechanism involved in the decomposition of MB dye. Fig. 14 (a) illustrates a schematic of the experimentation of the combined adsorption and photocatalytic degradation of MB dye with PPy-WO3 (0.3wt %) nanocomposite employed as catalysts in this study. The adsorption mechanism could be due to the electrostatic ionic interaction of the MB dye with the doped PPy-WO3 nanocomposite [50-52]. Fig. 14 (b& c) represents a schematic of the conductance and transfer of exciton pairs within the PPy-WO3 (0.3wt %) nanocomposite catalyst during the degradation of MB under UV light exposure. Each of the two components generates electron-hole pairs upon absorbing radiant energy. The h+ (positive holes) originating from WO3 quickly migrate to the valence band of Polypyrrole (PPy). This process forms *OH radicals by binding with molecules of H2O adsorbed on polypyrrole. Simultaneously, the photo-generated electrons continually transfer to the surface of WO3, where they react with dissolved oxygen in water, producing *O2 radicals. Reactive oxygen species and hydroxide radicals exhibit potent redox properties, which can effectively break down the intricate chemical bonds present in MB. Nevertheless, the presence of WO3 nanoparticles enhances the surface area of PPy, potentially facilitating a more significant interaction between the catalyst and light, thereby accelerating the oxidation process. Furthermore, WO3 serves as an electron transport layer, facilitating the transfer of light-induced electrons from Polypyrrole to WO3, effectively preventing the recombination of exciton pairs in PPy and thus enhancing photocatalytic efficacy. [38,42]. To find out the most reactive species that dominate the photodegradation process, scavenger trapping experiments were carried out using the scavenger p-benzoquinone (*O2- scavenger), isopropanol (*OH scavenger), silver nitrate (e- scavenger), and potassium iodide (h+ scavenger) and the results are depicted in Fig. 15. Without the scavenger, the degradation is 98.31%. The addition of p-benzoquinone (*O2- scavenger) contributes to a significant decrease in the photodegradation efficiency of the catalyst, i.e.,63.21%, and thus can be believed as the main active species for the degradation of the methylene blue dye [58]. The other active species contribute in the following order: *O2->*OH>e- >h+ with the degradation percentage as 63.21>71.60>75.88>88%.
Kinetics of Adsorption
The adsorption studies were carried out to determine the adsorption capacity of the catalyst PPy-WO3 (0.3wt.%) nanocomposite. The capacity of the material to adsorb qt (mg/g) was determined using Eq. 4.
Eq. 4
where:
C0 = initial concentration of the dye.
Ct = final concentration of the dye solution after time t
V = Volume of the dye solution (L)
m = mass of the adsorbent (g)
The following equation 5 presents the pseudo-second-order kinetic model proposed by Ho and McKay [43].
The data obtained from the experiment were modeled using the pseudo-second-order kinetic model to determine if this model provides a more accurate description of the adsorption process. The graphical representation of the pseudo-second-order kinetic model in Fig. 16 was derived by plotting the relationship between t/qt and time for the two materials investigated. By examining the kinetic parameters, particularly the Adj R2 value 0.97532, which is nearly equal to unity (1), the rate constant, which is 0.00204 min-1, and Pearson’s R-value, which is 0.98014, it can be concluded that pseudo-second-order kinetic model effectively describes the adsorption process of Methylene Blue Dye on the adsorbent PPy-WO3 (0.3 wt.%) nanocomposite. [37,44]
Comparative studies
The findings obtained in this investigation are compared with those from previous studies that employed other metal oxides and their composites for the photocatalytic degradation of Methylene Blue dye. Xiang Zhang and his colleagues reported 90% degradation with ZnO nanostructures for an MB dye concentration of 5ppm for a catalyst loading of 0.15g/L at 120 mins [53], Rania Farouq reported 75.81% with TiO2 for the photocatalytic degradation of an MB dye concentration of 5ppm for a catalyst loading of 1.0 g/L at 120 mins [39]. In contrast, our studies report 96.37% degradation at 120 mins with a catalyst loading of 1.6g/L. Abdel Messih and colleagues reported 96% degradation with Ag/ZnO nanoparticles of an MB dye concentration of 6ppm for a catalyst loading of 3.0 g/L at 240 mins [54]. Raviteja Surakasi reported 75.81% degradation with WO3 nanoparticles of an MB dye for a catalyst loading of 0.01 g/L at 150mins, whereas we reported 98.31% degradation with 1.6 g/L catalyst loading at 160mins [48]. Table. 6 represents WO3 and its composites for the degradation of MB [40,45-47,49]. The MB dye removal efficacy was comparable and, in some cases, higher when compared to the other composites. The preparation methodology is feasible, and the amount of WO3 used was less than other researchers used to synthesize WO3 composites.
Conclusions
The PPy-WO3 nanostructured materials were efficiently synthesized by an in-situ method for photocatalytic applications. The morphology of PPy-WO3 nanocomposites exhibited predominantly interconnected crystalline structures and compact agglomerates, which were more pronounced with increasing concentrations of WO3. This revealed the uniform dispersion of WO3 nanoparticles in the PPy-WO3 nanocomposites. The average crystallite size using the Scherrer equation was 3.4nm, and the grain size calculated from the FE-SEM images was 95.88nm, which showed usefulness in photocatalytic studies.
The electrical conductivity studies revealed higher electrical conductivity at room temperature than that reported in the literature.
The effect of various reaction parameters on the photocatalytic degradation indicated two-step mechanisms with a pseudo-second-order kinetics model for adsorption. These results showcase the capability of the fabricated nanocomposite as a highly efficient photocatalyst for eliminating MB dye and reinforcing its environmentally sustainable characteristics.
FINANCIAL SUPPORT
The researchers received no financial support for conducting, writing, and publishing this study.
CONFLICT OF INTEREST
The authors hereby declare that there is no conflict of interest.
Policy Regarding Research Data and Statements Availability
Researchers who opt to utilize the data outlined in the academic publications while safeguarding participants’ confidentiality will be granted unrestricted utilization of the resources and necessary raw data mentioned in the publication.
Author Contribution
“All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Nandini V. Iyer, Jayant Kher, and Shekhar Bhame. Nandini V. Iyer wrote the first draft of the manuscript, and all authors commented on previous versions. All authors read and approved the final manuscript.”
[8]. Chen J, Wang X, Huang Y, Lv S, Cao X, Yun J, Cao D, Adsorption removal of pollutant dyes in wastewater by nitrogen-doped porous carbons derived from natural leaves. Eng Sci 5(7) (2018),30-38,https://doi.org/10.30919/es8d666.
[10]. X. Chen, X. Liu, L. Zhu, X. Tao, X. Wang, One-step fabrication of novel MIL-53 (Fe, Al) for synergistic adsorption-photocatalytic degradation of tetracycline, Chemosphere 291(2021), 1330 32; https://doi.org/10.1016/j.chemosphere.2021.133032.
[11]. S. Chkirida, N. Zari, R.Achour, H. Hassoune, A. Lachehab, R. Bouhfd, Highly synergic adsorption/photocatalytic efficiency of Alginate/Bentonite impregnated TiO2 beads for wastewater treatment, J Photochem Photobiol A Chem 412 (2021),113215; https://doi.org/10.1016/j.jphotochem.2021.113215
[14]. Y. Liu, C. Hou, T. Jiao, J. Song, X. Zhang, R. Xing, Q. Peng, Self-assembled AgNP-containing nanocomposites constructed by electrospinning as efficient dye photocatalyst materials for wastewater treatment, Nanomaterials 8(1) (2018), 35; https://doi.org/10.3390/nano8010035
[22]. Cesar Quijada Special Issue: Conductive Polymers: Materials and Applications Reprinted from Materials 13(2020), 2344; https://doi.org/10.3390/ma13102344
[26] Mohanapriya, M. K., Deshmukh, Kalim, Ahamed, M. Basheer, Chidambaram, K., and Khadheer Pasha, S. K. Influence of Cerium Oxide (CeO2) Nanoparticles on the Structural, Morphological, Mechanical and Dielectric Properties of PVA/PPy Blend Nanocomposites. Materials Today: Proceedings, 3 (2016), 1864-1873; https://doi.org/10.1016/j.matpr.2016.04.086